This is a preprint.
Elapsed time since BNT162b2 vaccine and risk of SARS-CoV-2 infection in a large cohort
- PMID: 34401882
- PMCID: PMC8366798
- DOI: 10.1101/2021.08.03.21261496
Elapsed time since BNT162b2 vaccine and risk of SARS-CoV-2 infection in a large cohort
Update in
-
Elapsed time since BNT162b2 vaccine and risk of SARS-CoV-2 infection: test negative design study.BMJ. 2021 Nov 24;375:e067873. doi: 10.1136/bmj-2021-067873. BMJ. 2021. PMID: 34819275 Free PMC article.
Abstract
Importance: Israel was among the first countries to launch a large-scale COVID-19 vaccination campaign, and quickly vaccinated its population, achieving early control over the spread of the virus. However, the number of COVID-19 cases is now rapidly increasing, which may indicate that vaccine protection decreases over time.
Objective: To determine whether time elapsed since the second BNT162b2 messenger RNA (mRNA) vaccine (Pfizer-BioNTech) injection is significantly associated with the risk of post-vaccination COVID-19 infection.
Design: This is a retrospective cohort study performed in a large state-mandated health care organization in Israel.
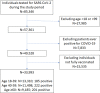
Participants: All fully vaccinated adults who have received a RT-PCR test between May 15, 2021 and July 26, 2021, at least two weeks after their second vaccine injection were included. Patients with a history of past COVID-19 infection were excluded.
Main outcome and measure: Positive result for the RT-PCR test.
Results: The cohort included 33,993 fully vaccinated adults, 49% women, with a mean age of 47 years (SD, 17 years), who received an RT-PCR test for SARS-CoV-2 during the study period. The median time between the second dose of the vaccine and the RT-PCR test was 146 days, interquartile range [121-167] days. 608 (1.8%) patients had positive test results. There was a significantly higher rate of positive results among patients who received their second vaccine dose at least 146 days before the RT-PCR test compared to patients who have received their vaccine less than 146 days before: odds ratio for infection was 3.00 for patients aged over 60 (95% CI 1.86-5.11); 2.29 for patients aged between 40 and 59 (95% CI 1.67-3.17); and 1.74 for patients aged between 18 and 39 (95% CI 1.27-2.37); P<0.001 in each age group.
Conclusions and relevance: In this large population study of patients tested for SARS-CoV-2 by RT-PCR following two doses of mRNA BNT162b2 vaccine, we observe a significant increase of the risk of infection in individuals who received their last vaccine dose since at least 146 days ago, particularly among patients older than 60.
Conflict of interest statement
Conflict of Interest Disclosures: None reported.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous