Mucosal AIDS virus transmission is enhanced by antiviral IgG isolated early in infection
- PMID: 34402452
- PMCID: PMC8631165
- DOI: 10.1097/QAD.0000000000003050
Mucosal AIDS virus transmission is enhanced by antiviral IgG isolated early in infection
Abstract
Objective: Antibody-dependent enhancement (ADE) affects host-virus dynamics in fundamentally different ways: i) enhancement of initial virus acquisition, and/or ii) increased disease progression/severity. Here we address the question whether anti-HIV-1 antibodies can enhance initial infection. While cell-culture experiments hinted at this possibility, in-vivo proof remained elusive.
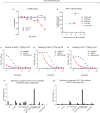
Design: We used passive immunization in nonhuman primates challenged with simian-human immunodeficiency virus (SHIV), a chimera expressing HIV-1 envelope. We purified IgG from rhesus monkeys with early-stage SHIV infection - before cross-neutralizing anti-HIV-1 antibodies had developed - and screened for maximal complement-mediated antibody-dependent enhancement (C'-ADE) of viral replication with a SHIV strain phylogenetically distinct from that harbored by IgG donor macaques. IgG fractions with maximal C'-ADE but lacking neutralization were combined to yield enhancing anti-SHIV IgG (enSHIVIG).
Results: We serially enrolled naive macaques (Group 1) to determine the minimal and 50% animal infectious doses required to establish persistent infection after intrarectal SHIV challenge. The first animal was inoculated with a 1 : 10 virus-stock dilution; after this animal's viral RNA load was >104copies/ml, the next macaque was challenged with 10x less virus, a process repeated until viremia no longer ensued. Group 2 was pretreated intravenously with enSHIVIG 24 h before SHIV challenge. Overall, Group 2 macaques required 3.4-fold less virus compared to controls (P = 0.002). This finding is consistent with enhanced susceptibility of the passively immunized animals to mucosal SHIV challenge.
Conclusion: These passive immunization data give proof of IgG-mediated enhanced virus acquisition after mucosal exposure - a potential concern for antibody-based AIDS vaccine development.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
-
- Guzman MG, Alvarez M, Halstead SB. Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: an historical perspective and role of antibody-dependent enhancement of infection. Arch Virol 2013; 158:1445–1459. - PubMed
-
- Kapikian AZ, Mitchell RH, Chanock RM, Shvedoff RA, Stewart CE. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol 1969; 89:405–421. - PubMed
-
- Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, et al. . Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol 1969; 89:422–434. - PubMed
-
- Polack FP, Hoffman SJ, Crujeiras G, Griffin DE. A role for nonprotective complement-fixing antibodies with low avidity for measles virus in atypical measles. Nat Med 2003; 9:1209–1213. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

