Biomechanics of the optic nerve head and sclera in canine glaucoma: A brief review
- PMID: 34402566
- PMCID: PMC9004594
- DOI: 10.1111/vop.12923
Biomechanics of the optic nerve head and sclera in canine glaucoma: A brief review
Abstract
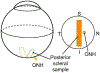
Glaucoma is a leading cause of irreversible blindness, a progressive optic neuropathy with retinal ganglion cell (RGC) death beginning in the optic nerve head (ONH). A primary risk factor for developing glaucoma is elevated intraocular pressure (IOP). Reducing IOP is the only treatment proven to be effective at delaying disease progression. Nevertheless, even when patients have their IOP reduced, the majority of them continue to lose vision. There are, in both humans and dogs, significant interindividual variabilities in susceptibilities to IOP-induced optic nerve damage. Vision loss progresses much more slowly in Beagles with open-angle glaucoma (OAG) caused by ADAMTS10 mutation. This can be attributed to the mutation-related altered ocular biomechanical properties. The principal site of optic nerve (ON) damage in glaucoma is the ONH. It is suggested that the biomechanical properties of the ONH and the surrounding peripapillary sclera (PPS) contribute to glaucoma development and progression. As far as the beneficial biomechanical properties of the ONH and PPS for a decreased susceptibility and slow progression of glaucoma, data are inconsistent and conflicting. Recent biomechanical studies on beagles with ADAMTS10 mutation demonstrated that the mutant dogs have mechanically weak posterior sclera. This weakness was associated with a reduced collagen density and a lower proportion of insoluble collagen. These changes, observed before glaucoma development, were considered intrinsic characteristics caused by the mutation rather than a secondary effect of IOP elevation. Further studies of ADAMTS10-OAG may elucidate the effects of altered biomechanical properties of ONH and PPS in determining the glaucoma progression.
Keywords: ADAMTS10; dog; neuroprotection; open-angle glaucoma; stiff; viscoelastic.
© 2021 American College of Veterinary Ophthalmologists.
Conflict of interest statement
CONFLICT OF INTEREST
None.
Figures
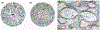
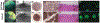

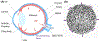
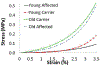
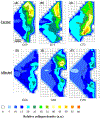
References
-
- Komaromy AM, Petersen-Jones SM. Genetics of canine primary glaucomas. Vet Clin N Am Small Anim Pract. 2015;45(6):1159–1182. v. - PubMed
-
- Plummer CE, Regnier A, Gelatte KN. The Canine Glaucomas. In: Gelatt KN, Gilger BC, Kern TJ, eds. Veterinary ophthalmology. 5th ed. Wiley-Blackwell Publishing; 2013:1050–1145.
-
- Curto EM, Gemensky-Metzler AJ, Chandler HL, Wilkie DA. Equine glaucoma: a histopathologic retrospective study (1999–2012). Vet Ophthalmol. 2014;17(5):334–342. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

