Th1 polarization defines the synovial fluid T cell compartment in oligoarticular juvenile idiopathic arthritis
- PMID: 34403374
- PMCID: PMC8492302
- DOI: 10.1172/jci.insight.149185
Th1 polarization defines the synovial fluid T cell compartment in oligoarticular juvenile idiopathic arthritis
Abstract
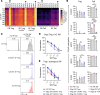
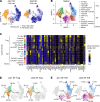
Oligoarticular juvenile idiopathic arthritis (oligo JIA) is the most common form of chronic inflammatory arthritis in children, yet the cause of this disease remains unknown. To understand immune responses in oligo JIA, we immunophenotyped synovial fluid T cells with flow cytometry, bulk RNA-Seq, single-cell RNA-Seq (scRNA-Seq), DNA methylation studies, and Treg suppression assays. In synovial fluid, CD4+, CD8+, and γδ T cells expressed Th1-related markers, whereas Th17 cells were not enriched. Th1 skewing was prominent in CD4+ T cells, including Tregs, and was associated with severe disease. Transcriptomic studies confirmed a Th1 signature in CD4+ T cells from synovial fluid. The regulatory gene expression signature was preserved in Tregs, even those exhibiting Th1 polarization. These Th1-like Tregs maintained Treg-specific methylation patterns and suppressive function, supporting the stability of this Treg population in the joint. Although synovial fluid CD4+ T cells displayed an overall Th1 phenotype, scRNA-Seq uncovered heterogeneous effector and regulatory subpopulations, including IFN-induced Tregs, peripheral helper T cells, and cytotoxic CD4+ T cells. In conclusion, oligo JIA is characterized by Th1 polarization that encompasses Tregs but does not compromise their regulatory identity. Targeting Th1-driven inflammation and augmenting Treg function may represent important therapeutic approaches in oligo JIA.
Keywords: Autoimmunity; Immunology; Rheumatology; T cells; Th1 response.
Conflict of interest statement
Figures





Comment in
-
Clonally expanded PD-1-expressing T cells are enriched in synovial fluid of juvenile idiopathic arthritis patients.Eur J Immunol. 2023 Jul;53(7):e2250162. doi: 10.1002/eji.202250162. Epub 2023 May 8. Eur J Immunol. 2023. PMID: 37086046
References
-
- Petty RE, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31(2):390–392. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

