SARS-CoV-2 (Covid-19) vaccines structure, mechanisms and effectiveness: A review
- PMID: 34403674
- PMCID: PMC8364403
- DOI: 10.1016/j.ijbiomac.2021.08.076
SARS-CoV-2 (Covid-19) vaccines structure, mechanisms and effectiveness: A review
Abstract
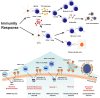
The world has been suffering from COVID-19 disease for more than a year, and it still has a high mortality rate. In addition to the need to minimize transmission of the virus through non-pharmacological measures such as the use of masks and social distance, many efforts are being made to develop a variety of vaccines to prevent the disease worldwide. So far, several vaccines have reached the final stages of safety and efficacy in various phases of clinical trials, and some, such as Moderna/NIAID and BioNTech/Pfizer, have reported very high safety and protection. The important point is that comparing different vaccines is not easy because there is no set standard for measuring neutralization. In this study, we have reviewed the common platforms of COVID-19 vaccines and tried to present the latest reports on the effectiveness of these vaccines.
Keywords: Adenovirus; Covid-19; Effectiveness; Inactivated; Infection; Sars-Cov-2; Vaccine.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
None to declare.
Figures
References
-
- Ozma M.A., Maroufi P., Khodadadi E., Köse S., Esposito I., Ganbarov K., Dao S., Esposito S., Dal T., Zeinalzadeh E. Clinical manifestation, diagnosis, prevention and control of SARS-CoV-2 (COVID-19) during the outbreak period. Infez. Med. 2020;28(2):153–165. - PubMed
-
- Najafi K., Maroufi P., Khodadadi E., Zeinalzadeh E., Ganbarov K., Asgharzadeh M., Kafil H.S. SARS-CoV-2 receptor ACE2 and molecular pathway to enter target cells during infection, reviews in medical. Microbiology. 2020
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous