Vaccine nationalism and the dynamics and control of SARS-CoV-2
- PMID: 34404735
- PMCID: PMC9835930
- DOI: 10.1126/science.abj7364
Vaccine nationalism and the dynamics and control of SARS-CoV-2
Abstract
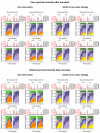
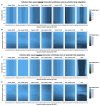
Vaccines provide powerful tools to mitigate the enormous public health and economic costs that the ongoing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic continues to exert globally, yet vaccine distribution remains unequal among countries. To examine the potential epidemiological and evolutionary impacts of “vaccine nationalism,” we extend previous models to include simple scenarios of stockpiling between two regions. In general, when vaccines are widely available and the immunity they confer is robust, sharing doses minimizes total cases across regions. A number of subtleties arise when the populations and transmission rates in each region differ, depending on evolutionary assumptions and vaccine availability. When the waning of natural immunity contributes most to evolutionary potential, sustained transmission in low-access regions results in an increased potential for antigenic evolution, which may result in the emergence of novel variants that affect epidemiological characteristics globally. Overall, our results stress the importance of rapid, equitable vaccine distribution for global control of the pandemic.
Figures



References
-
- Polack F. P., Thomas S. J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J. L., Pérez Marc G., Moreira E. D., Zerbini C., Bailey R., Swanson K. A., Roychoudhury S., Koury K., Li P., Kalina W. V., Cooper D., Frenck R. W. Jr., Hammitt L. L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D. B., Mather S., Dormitzer P. R., Şahin U., Jansen K. U., Gruber W. C., C4591001 Clinical Trial Group , Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N. Engl. J. Med. 383, 2603–2615 (2020). 10.1056/NEJMoa2034577 - DOI - PMC - PubMed
-
- Voysey M., Clemens S. A. C., Madhi S. A., Weckx L. Y., Folegatti P. M., Aley P. K., Angus B., Baillie V. L., Barnabas S. L., Bhorat Q. E., Bibi S., Briner C., Cicconi P., Collins A. M., Colin-Jones R., Cutland C. L., Darton T. C., Dheda K., Duncan C. J. A., Emary K. R. W., Ewer K. J., Fairlie L., Faust S. N., Feng S., Ferreira D. M., Finn A., Goodman A. L., Green C. M., Green C. A., Heath P. T., Hill C., Hill H., Hirsch I., Hodgson S. H. C., Izu A., Jackson S., Jenkin D., Joe C. C. D., Kerridge S., Koen A., Kwatra G., Lazarus R., Lawrie A. M., Lelliott A., Libri V., Lillie P. J., Mallory R., Mendes A. V. A., Milan E. P., Minassian A. M., McGregor A., Morrison H., Mujadidi Y. F., Nana A., O’Reilly P. J., Padayachee S. D., Pittella A., Plested E., Pollock K. M., Ramasamy M. N., Rhead S., Schwarzbold A. V., Singh N., Smith A., Song R., Snape M. D., Sprinz E., Sutherland R. K., Tarrant R., Thomson E. C., Török M. E., Toshner M., Turner D. P. J., Vekemans J., Villafana T. L., Watson M. E. E., Williams C. J., Douglas A. D., Hill A. V. S., Lambe T., Gilbert S. C., Pollard A. J., Oxford COVID Vaccine Trial Group , Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 397, 99–111 (2021). 10.1016/S0140-6736(20)32661-1 - DOI - PMC - PubMed
-
- Baden L. R., El Sahly H. M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S. A., Rouphael N., Creech C. B., McGettigan J., Khetan S., Segall N., Solis J., Brosz A., Fierro C., Schwartz H., Neuzil K., Corey L., Gilbert P., Janes H., Follmann D., Marovich M., Mascola J., Polakowski L., Ledgerwood J., Graham B. S., Bennett H., Pajon R., Knightly C., Leav B., Deng W., Zhou H., Han S., Ivarsson M., Miller J., Zaks T., COVE Study Group , Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384, 403–416 (2021). 10.1056/NEJMoa2035389 - DOI - PMC - PubMed
-
- Saad-Roy C. M., Wagner C. E., Baker R. E., Morris S. E., Farrar J., Graham A. L., Levin S. A., Mina M. J., Metcalf C. J. E., Grenfell B. T., Immune life history, vaccination, and the dynamics of SARS-CoV-2 over the next 5 years. Science 370, 811–818 (2020). 10.1126/science.abd7343 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

