Protective humoral and cellular immune responses to SARS-CoV-2 persist up to 1 year after recovery
- PMID: 34404803
- PMCID: PMC8370972
- DOI: 10.1038/s41467-021-25312-0
Protective humoral and cellular immune responses to SARS-CoV-2 persist up to 1 year after recovery
Abstract
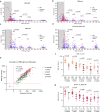
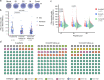
SARS-CoV-2 vaccination has been launched worldwide to build effective population-level immunity to curb the spread of this virus. The effectiveness and duration of protective immunity is a critical factor for public health. Here, we report the kinetics of the SARS-CoV-2 specific immune response in 204 individuals up to 1-year after recovery from COVID-19. RBD-IgG and full-length spike-IgG concentrations and serum neutralizing capacity decreases during the first 6-months, but is maintained stably up to 1-year after hospital discharge. Even individuals who had generated high IgG levels during early convalescent stages had IgG levels that had decreased to a similar level one year later. Notably, the RBD-IgG level positively correlates with serum neutralizing capacity, suggesting the representative role of RBD-IgG in predicting serum protection. Moreover, viral-specific cellular immune protection, including spike and nucleoprotein specific, persisted between 6 months and 12 months. Altogether, our study supports the persistence of viral-specific protective immunity over 1 year.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

