A broadly neutralizing humanized ACE2-targeting antibody against SARS-CoV-2 variants
- PMID: 34404805
- PMCID: PMC8371079
- DOI: 10.1038/s41467-021-25331-x
A broadly neutralizing humanized ACE2-targeting antibody against SARS-CoV-2 variants
Abstract
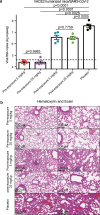
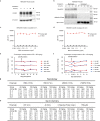
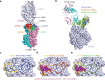
The successive emergences and accelerating spread of novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) lineages and evolved resistance to some ongoing clinical therapeutics increase the risks associated with the coronavirus disease 2019 (COVID-19) pandemic. An urgent intervention for broadly effective therapies to limit the morbidity and mortality of COVID-19 and future transmission events from SARS-related coronaviruses (SARSr-CoVs) is needed. Here, we isolate and humanize an angiotensin-converting enzyme-2 (ACE2)-blocking monoclonal antibody (MAb), named h11B11, which exhibits potent inhibitory activity against SARS-CoV and circulating global SARS-CoV-2 lineages. When administered therapeutically or prophylactically in the hACE2 mouse model, h11B11 alleviates and prevents SARS-CoV-2 replication and virus-induced pathological syndromes. No significant changes in blood pressure and hematology chemistry toxicology were observed after injections of multiple high dosages of h11B11 in cynomolgus monkeys. Analysis of the structures of the h11B11/ACE2 and receptor-binding domain (RBD)/ACE2 complexes shows hindrance and epitope competition of the MAb and RBD for the receptor. Together, these results suggest h11B11 as a potential therapeutic countermeasure against SARS-CoV, SARS-CoV-2, and escape variants.
© 2021. The Author(s).
Conflict of interest statement
Y.D., R.S., C.W., and J.Y. are listed as inventors on pending patent applications for h11B11. The other authors declare that they have no competing interests.
Figures


Similar articles
-
Characterization of a Broadly Neutralizing Monoclonal Antibody against SARS-CoV-2 Variants.Viruses. 2022 Jan 24;14(2):230. doi: 10.3390/v14020230. Viruses. 2022. PMID: 35215823 Free PMC article.
-
RBD-Fc-based COVID-19 vaccine candidate induces highly potent SARS-CoV-2 neutralizing antibody response.Signal Transduct Target Ther. 2020 Nov 27;5(1):282. doi: 10.1038/s41392-020-00402-5. Signal Transduct Target Ther. 2020. PMID: 33247109 Free PMC article.
-
A human antibody of potent efficacy against SARS-CoV-2 in rhesus macaques showed strong blocking activity to B.1.351.MAbs. 2021 Jan-Dec;13(1):1930636. doi: 10.1080/19420862.2021.1930636. MAbs. 2021. PMID: 34097570 Free PMC article.
-
Recovery scenario and immunity in COVID-19 disease: A new strategy to predict the potential of reinfection.J Adv Res. 2021 Jul;31:49-60. doi: 10.1016/j.jare.2020.12.013. Epub 2021 Jan 5. J Adv Res. 2021. PMID: 33520309 Free PMC article. Review.
-
SARS-CoV-2 evolution has increased resistance to monoclonal antibodies and first-generation COVID-19 vaccines: Is there a future therapeutic role for soluble ACE2 receptors for COVID-19?Antiviral Res. 2024 Jul;227:105894. doi: 10.1016/j.antiviral.2024.105894. Epub 2024 Apr 25. Antiviral Res. 2024. PMID: 38677595 Review.
Cited by
-
Cell-based passive immunization for protection against SARS-CoV-2 infection.Stem Cell Res Ther. 2023 Nov 6;14(1):318. doi: 10.1186/s13287-023-03556-5. Stem Cell Res Ther. 2023. PMID: 37932852 Free PMC article.
-
Topical TMPRSS2 inhibition prevents SARS-CoV-2 infection in differentiated human airway cultures.Life Sci Alliance. 2022 Feb 2;5(4):e202101116. doi: 10.26508/lsa.202101116. Print 2022 Apr. Life Sci Alliance. 2022. PMID: 35110354 Free PMC article.
-
Design and Characterization of Bispecific and Trispecific Antibodies Targeting SARS-CoV-2.Vaccines (Basel). 2025 Feb 28;13(3):255. doi: 10.3390/vaccines13030255. Vaccines (Basel). 2025. PMID: 40266148 Free PMC article.
-
Sarbecovirus RBD indels and specific residues dictating multi-species ACE2 adaptiveness.Nat Commun. 2024 Oct 14;15(1):8869. doi: 10.1038/s41467-024-53029-3. Nat Commun. 2024. PMID: 39402048 Free PMC article.
-
Nanobodies with cross-neutralizing activity provide prominent therapeutic efficacy in mild and severe COVID-19 rodent models.Virol Sin. 2023 Oct;38(5):787-800. doi: 10.1016/j.virs.2023.07.003. Epub 2023 Jul 8. Virol Sin. 2023. PMID: 37423308 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

