Access to cationic polyhedral carboranes via dynamic cage surgery with N-heterocyclic carbenes
- PMID: 34404809
- PMCID: PMC8371172
- DOI: 10.1038/s41467-021-25277-0
Access to cationic polyhedral carboranes via dynamic cage surgery with N-heterocyclic carbenes
Abstract
Polyhedral boranes and heteroboranes appear almost exclusively as neutral or anionic species, while the cationic ones are protonated at exoskeletal heteroatoms or they are instable. Here we report the reactivity of 10-vertex closo-dicarbadecaboranes with one or two equivalents of N-heterocyclic carbene to 10-vertex nido mono- and/or bis-carbene adducts, respectively. These complexes easily undergo a reaction with HCl to give cages of stable and water soluble 10-vertex nido-type cations with protonation in the form of a BHB bridge or 10-vertex closo-type cations containing one carbene ligand when originating from closo-1,10-dicarbadecaborane. The reaction of a 10-vertex nido mono-carbene adduct with phosphorus trichloride gives nido-11-vertex 2-phospha-7,8-dicarbaundecaborane, which undergoes an oxidation of the phosphorus atom to P = O, while the product of a bis-carbene adduct reaction is best described as a distorted C2B6H8 fragment bridged by the (BH)2PCl2+ moiety.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
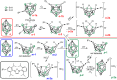
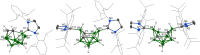

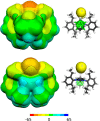
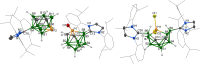
References
-
- Grimes RN. Carboranes. 3rd edn. Academic Press; 2016.
-
- Hosmane NS. Boron Science: New Technologies and Applications. CRC Press; 2012.
-
- Hnyk D, McKee M. Boron: The Fifth Element. 1st edn. Springer; 2015.
-
- Brown HC, Heim P. Diborane as a mild reducing agent for the conversion of primary, secondary, and tertiary amides into the corresponding amines. J. Am. Chem. Soc. 1964;86:3566–3567. doi: 10.1021/ja01071a037. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

