Results of a Pilot Study on the Safety and Early Efficacy of Novel Polymethyl Methacrylate Microspheres and a Hyaluronic Acid Device for the Treatment of Low Back Pain Caused by Degenerative and Diseased Intervertebral Discs of the Lumbar Spine
- PMID: 34405067
- PMCID: PMC8353671
- DOI: 10.7759/cureus.16308
Results of a Pilot Study on the Safety and Early Efficacy of Novel Polymethyl Methacrylate Microspheres and a Hyaluronic Acid Device for the Treatment of Low Back Pain Caused by Degenerative and Diseased Intervertebral Discs of the Lumbar Spine
Abstract
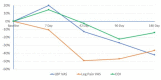
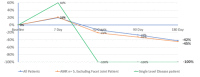
Background Low back pain (LBP) costs the healthcare system billions of dollars each year. Intervertebral disc (IVD) degeneration is a significant cause of LBP, due to structural defects, biomechanical instability, and inflammation. First-line therapy for patients with LBP includes physical therapy, medication, and steroid injections. DiscSealTM was developed to provide patients who are refractory to first-line therapy with a minimally invasive treatment alternative to invasive surgical procedures. The product is a combination of poly-methyl methacrylate (PMMA) microspheres in hyaluronic acid (HA) that is injected under modified discography into the IVD. Methods Two pain specialist centers in Australia recruited eligible participants who were followed up for 180 days post-procedure. The procedure was conducted using a modified discography technique. Low back and leg pain was reported using the Visual Analogue Scale (VAS) while other endpoints included were Oswestry Disability Index (ODI), Clinician and Patient Global Impact of Change, and Patient Rating of Overall Health Status. The general analytical approach for all endpoints was descriptive in nature and 95% confidence intervals of means were estimated. Results The pilot study achieved its primary objective which was an absence of peri-treatment or post-treatment device-related Serious Adverse Events (SAE) during the first 90 days. There were no device-related serious adverse events recorded throughout the study. The mean LBP percentage change from baseline at 90 and 180 days was -27.0% and -42.3% respectively. The mean ODI percentage change from baseline at 90 and 180 days was -22.3 and -14.2% respectively. End of study improvements shows a 67.8% (20.83) increase in Overall Health Status, as well as positive results for Participant and Clinician Global Impact of Change. These results were achieved based on treating one diseased IVD, although 83.3% of patients were diagnosed with multiple diseased IVDs. Conclusions The results from this pilot study showed that DiscSealTM is safe and well-tolerated. Early efficacy shows that DiscSealTM may be a promising treatment option for people suffering from discogenic LBP that have not responded to first-line treatment options. A larger, statistically powered study where all diseased discs are treated should be completed to validate the promising results from this early feasibility study.
Keywords: chronic low back pain (clbp); clinical trial; degenerative disc disease; discogenic back pain; first in human.
Copyright © 2021, Yu et al.
Conflict of interest statement
The investigational product, DiscSeal is patented. Dr James Yu, Dr Alex Gavino and Dr Masego Johnstone are employees of Australian Medical Research. Australian Medical Research was paid by SpineOvations, Inc. to conduct the study. Dr Paul Verrills and Dr Jacqui Young are employees of Metro Pain Group. Metro Pain Group was paid by SpineOvations, Inc. to conduct the study. No individual authors were paid directly to conduct the study.
Figures
References
-
- Estimating cost of care for patients with acute low back pain: a retrospective review of patient records. Crow WT, Willis DR. https://pubmed.ncbi.nlm.nih.gov/19369510/ J Am Osteopath Assoc. 2009;109:229–233. - PubMed
-
- What are the most common conditions in primary care? Systematic review. Finley CR, Chan DS, Garrison S, et al. https://www.cfp.ca/content/64/11/832.short. Can Fam Physician. 2018;64:832–840. - PMC - PubMed
-
- Priority diseases and reasons for inclusion. World Health Organisation. [Jul;2021 ];Organisation WH. https://www.who.int/medicines/areas/priority_medicines/Ch6_24LBP.pdf World Health Organisation. 6.24:1–3.
-
- Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Qaseem A, Wilt TJ, McLean RM, Forciea MA. Ann Intern Med. 2017;166:514–530. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
