Immune complexes, innate immunity, and NETosis in ChAdOx1 vaccine-induced thrombocytopenia
- PMID: 34405870
- PMCID: PMC8385969
- DOI: 10.1093/eurheartj/ehab506
Immune complexes, innate immunity, and NETosis in ChAdOx1 vaccine-induced thrombocytopenia
Abstract
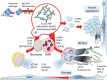
Aims: We recently reported five cases of vaccine-induced immune thrombotic thrombocytopenia (VITT) 7-10 days after receiving the first dose of the ChAdOx1 nCoV-19 adenoviral vector vaccine against corona virus disease 2019 (COVID-19). We aimed to investigate the pathogenic immunological responses operating in these patients.
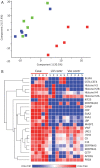
Methods and results: We assessed circulating inflammatory markers by immune assays and immune cell phenotyping by flow cytometry analyses and performed immunoprecipitation with anti-platelet factor (PF)4 antibody in plasma samples followed by mass spectrometry from all five patients. A thrombus was retrieved from the sinus sagittal superior of one patient and analysed by immunohistochemistry and flow cytometry. Precipitated immune complexes revealed multiple innate immune pathway triggers for platelet and leucocyte activation. Plasma contained increased levels of innate immune response cytokines and markers of systemic inflammation, extensive degranulation of neutrophils, and tissue and endothelial damage. Blood analyses showed activation of neutrophils and increased levels of circulating H3Cit, dsDNA, and myeloperoxidase-DNA complex. The thrombus had extensive infiltration of neutrophils, formation of neutrophil extracellular traps (NETs), and IgG deposits.
Conclusions: The results show that anti-PF4/polyanion IgG-mediated thrombus formation in VITT patients is accompanied by a massive innate immune activation and particularly the fulminant activation of neutrophils including NETosis. These results provide novel data on the immune response in this rare adenoviral vector-induced VITT.
Keywords: Immune activation; Neutrophils; Thrombus; Vaccine-induced immune thrombotic thrombocytopenia.
© The Author(s) 2021. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures



Comment in
-
Thromboinflammatory findings and clinical predictors of mortality in vaccine-induced immune thrombotic thrombocytopenia.Eur Heart J. 2021 Oct 14;42(39):4073-4076. doi: 10.1093/eurheartj/ehab585. Eur Heart J. 2021. PMID: 34545405 No abstract available.
References
-
- Norwegian Institute of Public Health. COVID 19 Ukerapport—uke 10. 2021. https://www.fhi.no/contentassets/8a971e7b0a3c4a06bdbf381ab52e6157/vedleg... August 2021).
-
- Norwegian Institute of Public Health. Vaccination with AstraZeneca Vaccine against COVID-19 Put on Hold. 2021. https://www.fhi.no/en/news/2021/vaksinasjon-med-astrazeneca-vaksinen-mot....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

