Protein Assembly by Design
- PMID: 34405992
- PMCID: PMC9148388
- DOI: 10.1021/acs.chemrev.1c00308
Protein Assembly by Design
Abstract
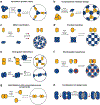
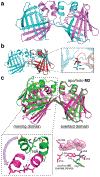
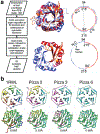
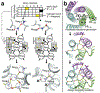
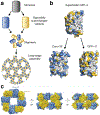
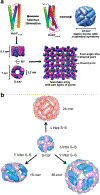
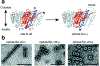
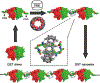
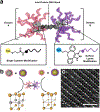
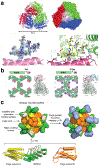
Proteins are nature's primary building blocks for the construction of sophisticated molecular machines and dynamic materials, ranging from protein complexes such as photosystem II and nitrogenase that drive biogeochemical cycles to cytoskeletal assemblies and muscle fibers for motion. Such natural systems have inspired extensive efforts in the rational design of artificial protein assemblies in the last two decades. As molecular building blocks, proteins are highly complex, in terms of both their three-dimensional structures and chemical compositions. To enable control over the self-assembly of such complex molecules, scientists have devised many creative strategies by combining tools and principles of experimental and computational biophysics, supramolecular chemistry, inorganic chemistry, materials science, and polymer chemistry, among others. Owing to these innovative strategies, what started as a purely structure-building exercise two decades ago has, in short order, led to artificial protein assemblies with unprecedented structures and functions and protein-based materials with unusual properties. Our goal in this review is to give an overview of this exciting and highly interdisciplinary area of research, first outlining the design strategies and tools that have been devised for controlling protein self-assembly, then describing the diverse structures of artificial protein assemblies, and finally highlighting the emergent properties and functions of these assemblies.
Figures




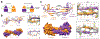

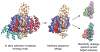

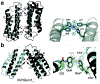
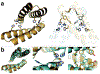
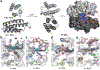

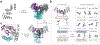

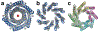









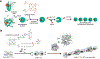

























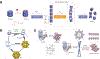

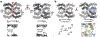






References
-
- Miller SL The Formation of Organic Compounds on the Primitive Earth. Ann. N. Y. Acad. Sci 1957, 69, 260–275 - PubMed
-
- Miller SL; Urey HC Organic Compound Synthesis on the Primitive Earth. Science 1959, 130, 245–251 - PubMed
-
- Miller SL; Urey HC; Oro J Origin of Organic Compounds on the Primitive Earth and in Meteorites. J. Mol. Evol 1976, 9, 59–72 - PubMed
-
- Joyce GF Rna Evolution and the Origins of Life. Nature 1989, 338, 217–224 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

