High Salinity Inhibits Soil Bacterial Community Mediating Nitrogen Cycling
- PMID: 34406835
- PMCID: PMC8516042
- DOI: 10.1128/AEM.01366-21
High Salinity Inhibits Soil Bacterial Community Mediating Nitrogen Cycling
Abstract
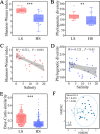
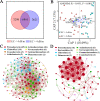
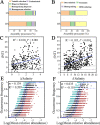
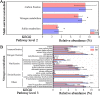
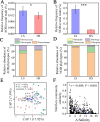
Salinization is considered a major threat to soil fertility and agricultural productivity throughout the world. Soil microbes play a crucial role in maintaining ecosystem stability and function (e.g., nitrogen cycling). However, the response of bacterial community composition and community-level function to soil salinity remains uncertain. Here, we used multiple statistical analyses to assess the effect of high salinity on bacterial community composition and potential metabolism function in the agricultural ecosystem. Results showed that high salinity significantly altered both bacterial alpha (Shannon-Wiener index and phylogenetic diversity) and beta diversity. Salinity, total nitrogen (TN), and soil organic matter (SOM) were the vital environmental factors shaping bacterial community composition. The relative abundance of Actinobacteria, Chloroflexi, Acidobacteria, and Planctomycetes decreased with salinity, whereas Proteobacteria and Bacteroidetes increased with salinity. The modularity and the ratio of negative to positive links remarkedly decreased, indicating that high salinity destabilized bacterial networks. Variable selection, which belongs to deterministic processes, mediated bacterial community assembly within the saline soils. Function prediction results showed that the key nitrogen metabolism (e.g., ammonification, nitrogen fixation, nitrification, and denitrification processes) was inhibited in high salinity habitats. MiSeq sequencing of 16S rRNA genes revealed that the abundance and composition of the nitrifying community were influenced by high salinity. The consistency of function prediction and experimental verification demonstrated that high salinity inhibited soil bacterial community mediating nitrogen cycling. Our study provides strong evidence for a salinity effect on the bacterial community composition and key metabolism function, which could help us understand how soil microbes respond to ongoing environment perturbation. IMPORTANCE Revealing the response of the soil bacterial community to external environmental disturbances is an important but poorly understood topic in microbial ecology. In this study, we evaluated the effect of high salinity on the bacterial community composition and key biogeochemical processes in salinized agricultural soils (0.22 to 19.98 dS m-1). Our results showed that high salinity significantly decreased bacterial diversity, altered bacterial community composition, and destabilized the bacterial network. Moreover, variable selection (61% to 66%) mediated bacterial community assembly within the saline soils. Functional prediction combined with microbiological verification proved that high salinity inhibited soil bacterial community mediating nitrogen turnover. Understanding the impact of salinity on soil bacterial community is of great significance for managing saline soils and maintaining a healthy ecosystem.
Keywords: bacterial community; community assembly; functional prediction; network stability; nitrogen cycling; soil salinization.
Figures

References
-
- Szabolcs I. 1989. Salt-affected soils. CRC Press, Inc. Boca Raton, FL.
-
- Richards LA. 1954. Diagnosis and improvement of saline and alkali soils. United States Department of Agriculture, Washington, DC.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

