Syncytialization alters the extracellular matrix and barrier function of placental trophoblasts
- PMID: 34406903
- PMCID: PMC8560385
- DOI: 10.1152/ajpcell.00177.2021
Syncytialization alters the extracellular matrix and barrier function of placental trophoblasts
Abstract
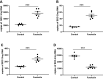
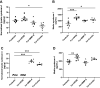
The human placenta is of vital importance for proper nutrient and waste exchange, immune regulation, and overall fetal health and growth. Specifically, the extracellular matrix (ECM) of placental syncytiotrophoblasts, which extends outward from the placental chorionic villi into maternal blood, acts on a molecular level to regulate and maintain this barrier. Importantly, placental barrier dysfunction has been linked to diseases of pregnancy such as preeclampsia and intrauterine growth restriction. To help facilitate our understanding of the interface and develop therapeutics to repair or prevent dysfunction of the placental barrier, in vitro models of the placental ECM would be of great value. In this study, we aimed to characterize the ECM of an in vitro model of the placental barrier using syncytialized BeWo choriocarcinoma cells. Syncytialization caused a marked change in syndecans, integral proteoglycans of the ECM, which matched observations of in vivo placental ECM. Syndecan-1 expression increased greatly and predominated the other variants. Barrier function of the ECM, as measured by electric cell-substrate impedance sensing (ECIS), increased significantly during and after syncytialization, whereas the ability of THP-1 monocytes to adhere to syncytialized BeWos was greatly reduced compared with nonsyncytialized controls. Furthermore, ECIS measurements indicated that ECM degradation with matrix metalloproteinase-9 (MMP-9), but not heparanase, decreased barrier function. This decrease in ECIS-measured barrier function was not associated with any changes in THP-1 adherence to syncytialized BeWos treated with heparanase or MMP-9. Thus, syncytialization of BeWos provides a physiologically accurate placental ECM with a barrier function matching that seen in vivo.
Keywords: barrier; extracellular matrix; placenta; syncytialize; trophoblast.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

