Adjuvanted recombinant zoster vaccine in adult autologous stem cell transplant recipients: polyfunctional immune responses and lessons for clinical practice
- PMID: 34406911
- PMCID: PMC8828160
- DOI: 10.1080/21645515.2021.1953346
Adjuvanted recombinant zoster vaccine in adult autologous stem cell transplant recipients: polyfunctional immune responses and lessons for clinical practice
Abstract
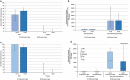
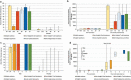
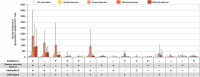
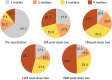
Immunocompromised individuals, particularly autologous hematopoietic stem cell transplant (auHSCT) recipients, are at high risk for herpes zoster (HZ). We provide an in-depth description of humoral and cell-mediated immune (CMI) responses by age (protocol-defined) or underlying disease (post-hoc) as well as efficacy by underlying disease (post-hoc) of the adjuvanted recombinant zoster vaccine (RZV) in a randomized observer-blind phase III trial (ZOE-HSCT, NCT01610414). 1846 adult auHSCT recipients were randomized to receive a first dose of either RZV or placebo 50-70 days post-auHSCT, followed by the second dose at 1-2 months (M) later. In cohorts of 114-1721 participants, at 1 M post-second vaccine dose: Anti-gE antibody geometric mean concentrations (GMCs) and median gE-specific CD4[2+] T-cell frequencies (CD4 T cells expressing ≥2 of four assessed activation markers) were similar between 18-49 and ≥50-year-olds. Despite lower anti-gE antibody GMCs in non-Hodgkin B-cell lymphoma (NHBCL) patients, CD4[2+] T-cell frequencies were similar between NHBCL and other underlying diseases. The proportion of polyfunctional CD4 T cells increased over time, accounting for 79.6% of gE-specific CD4 T cells at 24 M post-dose two. Vaccine efficacy against HZ ranged between 42.5% and 82.5% across underlying diseases and was statistically significant in NHBCL and multiple myeloma patients. In conclusion, two RZV doses administered early post-auHSCT induced robust, persistent, and polyfunctional gE-specific immune responses. Efficacy against HZ was also high in NHBCL patients despite the lower humoral response.
Keywords: Autologous hematopoietic stem cell transplant; adjuvanted recombinant zoster vaccine; cell-mediated immunity; humoral immune response; polyfunctionality; vaccine efficacy.
Plain language summary
PLAIN LANGUAGE SUMMARYWhat is the context?After haematopoietic stem cell transplantation, patients have impaired immunity from conditioning chemotherapy regimens, often exacerbated by underlying diseases, putting them at high risk of developing herpes zoster. In this population, antiviral prophylaxis is the current standard of care to reduce herpes zoster risk. Vaccination provides an additional means to prevent herpes zoster. Live-attenuated vaccines are generally contraindicated in immunocompromised patients. A non-live, adjuvanted recombinant zoster vaccine (RZV, Shingrix, GSK), has been approved for use in adults ≥50 years of age in the European Union, United States, Canada, Australia, Japan, and China. This vaccine is highly efficacious at preventing herpes zoster in adults over 50 years of age, as demonstrated in large, placebo-controlled randomised trials. Importantly, Shingrix use is not contraindicated in immunocompromised conditions, and was found to be highly efficacious in adults who had recently undergone autologous haematopoietic stem cell transplant.What is new?In autologous haematopoietic stem cell transplant recipients in whom Shingrix has demonstrated efficacy, two doses elicited high and persistent immune responses. Date presented here further support our understanding of the impact of specific factors such as age or underlying diseases on the vaccine’s effect in the population studied, as well as the characteristics of the elicited cell-mediated immune responses.What is the impact?These results indicate that Shingrix, given shortly after haematopoietic stem cell transplant, can induce robust immune responses and reduce the risk of herpes zoster, even in individuals with immunosuppression due to underlying disease and/or use of immunosuppressive therapies, regardless of age or underlying disease.
Conflict of interest statement
The authors declare the following financial relationships: During the conduct of the study, CA, and EAS report grants from the GSK group of companies (GSK); VJA reports Phase III study compensation from GSK to the research institute; and KMS reports a grant and personal fees from GSK and a grant from NIAID and NIH awarded to Duke University. Outside the submitted work, VJA reports study grants from Pfizer, and personal fees from Astellas, MSD, Pfizer, Roche, and Unimedic; AGr has served on the Advisory Boards of Bristol Myers Squibb, Gilead, MSD, Novartis, Roche, and Takeda; AGu reports personal fees from Celgene, Takeda, Janssen-Cilag, Novartis, Teva, and Jazz; and KMS reports personal fees from Kiadis Pharmaceutical, and Roche Genentech. During the design, initiation, conduct of the study and/or interpretation of the data, AB, AFD, EDP, MEI, TCH, LO, BS, and AES were employees of GSK. They also own shares in the GSK group of companies. AB, AFD, and LO are currently employed by the Bill & Melinda Gates Medical Research Institute, Mithra Pharmaceuticals, and CureVac AG, respectively. TCH and LO are inventors on a patent owned by GSK and relevant to RZV. Outside the submitted work, TCH was a paid consultant for GSK. AAA, AJCB, RB, CC, AC, HE, APGR, IJ, JYK, AL, MPZ, TCS, UMS, FV, LYSS, and PZ have no conflicting financial relationships to disclose. The authors declare no other non-financial relationships and activities or conflicts of interest.
Figures


References
-
- Oxman MN. Herpes zoster pathogenesis and cell-mediated immunity and immunosenescence. J Am Osteopath Assoc. 2009;109:S13–17. - PubMed
-
- Sahoo F, Hill JA, Xie H, Leisenring W, Yi J, Goyal S, Kimball LE, Lee I, Seo S, Davis C, et al. Herpes zoster in autologous hematopoietic cell transplant recipients in the era of acyclovir or valacyclovir prophylaxis and novel treatment and maintenance therapies. Biol Blood Marrow Transplant. 2017;23(3):505–11. doi:10.1016/j.bbmt.2016.12.620. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
