Rapid Detection of Anti-SARS-CoV-2 Antibody Using a Selenium Nanoparticle-Based Lateral Flow Immunoassay
- PMID: 34406945
- PMCID: PMC8905607
- DOI: 10.1109/TNB.2021.3105662
Rapid Detection of Anti-SARS-CoV-2 Antibody Using a Selenium Nanoparticle-Based Lateral Flow Immunoassay
Abstract
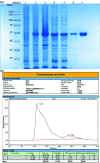
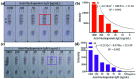
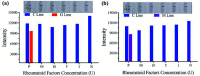
Coronavirus disease 2019 is an infectious disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). SARS-CoV-2 is highly transmissible. Early and rapid testing is necessary to effectively prevent and control the outbreak. Detection of SARS-CoV-2 antibodies with lateral flow immunoassay can achieve this goal. In this study, SARS-CoV-2 nucleoprotein (NP) was expressed and purified. We used the selenium nanoparticle as the labeling probe coupled with the NP to prepare an antibody (IgM and IgG) detection kit. The detection limit, cross reaction, sensitivity and specificity of the kit is verified. Separate detection of IgM and IgG, such as in this assay, was performed in order to reduce mutual interference and improve the accuracy of the test results.The final purity of NP was 91.83%. Selenium nanoparticle and NP successfully combined with stable effect. The LOD of the kit was 20 ng/mL for anti-NP IgG and 60 ng/mL for anti-NP IgM, respectively. The kit does not cross reaction with RF. The sensitivity of the kit was 94.74% and the specificity was 96.23%. The assay kit does not require any special device for reading the results and the readout is a simple color change that can be evaluated with the naked eye. This kit is suitable for rapid and real-time detection of the SARS-CoV-2 antibody IgG and IgM.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

