Antitumor effects of chloroquine/hydroxychloroquine mediated by inhibition of the NF-κB signaling pathway through abrogation of autophagic p47 degradation in adult T-cell leukemia/lymphoma cells
- PMID: 34407152
- PMCID: PMC8372904
- DOI: 10.1371/journal.pone.0256320
Antitumor effects of chloroquine/hydroxychloroquine mediated by inhibition of the NF-κB signaling pathway through abrogation of autophagic p47 degradation in adult T-cell leukemia/lymphoma cells
Abstract
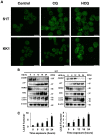
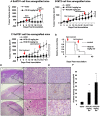
Adult T-cell leukemia/lymphoma (ATLL) originates from human T-cell leukemia virus type 1 (HTLV-1) infection due to the activation of the nuclear factor-κB (NF-κB) signaling pathway to maintain proliferation and survival. An important mechanism of the activated NF-κB signaling pathway in ATLL is the activation of the macroautophagy (herafter referred to as autophagy in the remainder of this manuscript)-lysosomal degradation of p47 (NSFL1C), a negative regulator of the NF-κB pathway. Therefore, we considered the use of chloroquine (CQ) or hydroxychloroquine (HCQ) (CQ/HCQ) as an autophagy inhibitor to treat ATLL; these drugs were originally approved by the FDA as antimalarial drugs and have recently been used to treat autoimmune diseases, such as systemic lupus erythematosus (SLE). In this paper, we determined the therapeutic efficacy of CQ/HCQ, as NF-κB inhibitors, in ATLL mediated by blockade of p47 degradation. Administration of CQ/HCQ to ATLL cell lines and primary ATLL cells induced cell growth inhibition in a dose-dependent manner, and the majority of cells underwent apoptosis after CQ administration. As to the molecular mechanism, autophagy was inhibited in CQ-treated ATLL cells, and activation of the NF-κB pathway was suppressed with the restoration of the p47 level. When the antitumor effect of CQ/HCQ was examined using immunodeficient mice transplanted with ATLL cell lines, CQ/HCQ significantly suppressed tumor growth and improved the survival rate in the ATLL xenograft mouse model. Importantly, HCQ selectively induced ATLL cell death in the ATLL xenograft mouse model at the dose used to treat SLE. Taken together, our results suggest that the inhibition of autophagy by CQ/HCQ may become a novel and effective strategy for the treatment of ATLL.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

