A novel anti-c-Kit antibody-drug conjugate to treat wild-type and activating-mutant c-Kit-positive tumors
- PMID: 34407310
- PMCID: PMC8936518
- DOI: 10.1002/1878-0261.13084
A novel anti-c-Kit antibody-drug conjugate to treat wild-type and activating-mutant c-Kit-positive tumors
Abstract
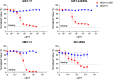
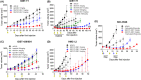
c-Kit overexpression and activating mutations, which are reported in various cancers, including gastrointestinal stromal tumor (GIST), small-cell lung cancer (SCLC), acute myeloid leukemia, acral melanoma, and systemic mastocytosis (SM), confer resistance to tyrosine kinase inhibitors (TKIs). To overcome TKI resistance, an anti-c-Kit antibody-drug conjugate was developed in this study to treat wild-type and mutant c-Kit-positive cancers. NN2101, a fully human IgG1, was conjugated to DM1, a microtubule inhibitor, through N-succinimidyl-4-(N-maleimidomethyl) cyclohexane-1-carboxylate (SMCC) (to give NN2101-DM1). The antitumor activity of NN2101-DM1 was evaluated in vitro and in vivo using various cancer cell lines. NN2101-DM1 exhibited potent growth-inhibitory activities against c-Kit-positive cancer cell lines. In a mouse xenograft model, NN2101-DM1 exhibited potent growth-inhibitory activities against imatinib-resistant GIST and SM cells. In addition, NN2101-DM1 exhibited a significantly higher anti-cancer effect than carboplatin/etoposide against SCLC cells where c-Kit does not mediate cancer pathogenesis. Furthermore, the combination of NN2101-DM1 with imatinib in imatinib-sensitive GIST cells induced complete remission compared with treatment with NN2101-DM1 or imatinib alone in mouse xenograft models. These results suggest that NN2101-DM1 is a potential therapeutic agent for wild-type and mutant c-Kit-positive cancers.
Keywords: antibody-drug conjugate; c-Kit; imatinib-resistant cancer; stem cell factor; tyrosine kinase inhibitor.
© 2021 The Authors. Molecular Oncology published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
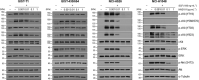
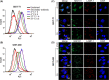
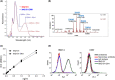
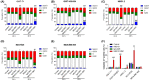
References
-
- Yasuda A, Sawai H, Takahashi H, Ochi N, Matsuo Y, Funahashi H, Sato M, Okada Y, Takeyama H & Manabe T (2007) Stem cell factor/c‐kit receptor signaling enhances the proliferation and invasion of colorectal cancer cells through the PI3K/Akt pathway. Dig Dis Sci 52, 2292–2300. - PubMed
-
- Broudy VC (1997) Stem cell factor and hematopoiesis. Blood 90, 1345–1364. - PubMed
-
- Lennartsson J & Ronnstrand L (2012) Stem cell factor receptor/c‐Kit: from basic science to clinical implications. Physiol Rev 92, 1619–1649. - PubMed
-
- Bougherara H, Subra F, Crepin R, Tauc P, Auclair C & Poul MA (2009) The aberrant localization of oncogenic kit tyrosine kinase receptor mutants is reversed on specific inhibitory treatment. Mol Cancer Res 7, 1525–1533. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

