Interactions between etonogestrel-releasing contraceptive implant and 3 antiretroviral regimens
- PMID: 34407424
- PMCID: PMC8678338
- DOI: 10.1016/j.contraception.2021.08.006
Interactions between etonogestrel-releasing contraceptive implant and 3 antiretroviral regimens
Abstract
Objectives: Long-acting reversible contraceptives are effective contraceptives for women with HIV, but there are limited data on etonogestrel implant and antiretroviral therapy pharmacokinetic drug-drug interactions. We evaluated etonogestrel/antiretroviral therapy drug-drug interactions, and the effects of etonogestrel on ritonavir-boosted-atazanavir, ritonavir-boosted-lopinavir, and efavirenz pharmacokinetics.
Study design: We enrolled postpartum women using etonogestrel implants and receiving ritonavir-boosted-atazanavir, ritonavir-boosted-lopinavir, or efavirenz-based regimens between 2012 and 2015. Etonogestrel implants were inserted 2 to 12 weeks postpartum. We performed pharmacokinetic sampling pre-etonogestrel insertion and 6 to 7 weeks postinsertion. We measured antiretroviral concentrations pre and postetonogestrel insertion, and compared etonogestrel concentrations between antiretroviral regimens. We considered a minimum serum etonogestrel concentration of 90 pg/mL adequate for ovulation suppression.
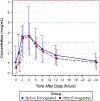
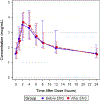
Results: We collected pharmacokinetic data for 74 postpartum women, 22 on ritonavir-boosted-atazanavir, 26 on ritonavir-boosted-lopinavir, and 26 on efavirenz. The median serum concentrations of etonogestrel when co-administered were highest with etonogestrel/ritonavir-boosted-atazanavir (604 pg/mL) and etonogestrel/ritonavir-boosted-lopinavir (428 pg/mL), and lowest with etonogestrel/efavirenz (125 pg/mL); p < 0.001. Minimum concentration (Cmin) of ritonavir-boosted-atazanavir and ritonavir-boosted-lopinavir were lower after etonogestrel implant insertion, but overall exposure, predose concentrations, clearance, and half-lives were unchanged. We found no significant change in efavirenz exposure after etonogestrel insertion.
Conclusions: Unlike efavirenz, ritonavir-boosted-atazanavir and ritonavir-boosted-lopinavir were not associated with significant decreases in etonogestrel concentrations. Efavirenz was associated with a significant decrease in etonogestrel concentrations.
Implications: The findings demonstrate no interactions between etonogestrel and ritonavir-boosted-lopinavir or ritonavir-boosted-atazanavir, but confirm the decreased efficacy of etonogestrel with efavirenz-based antiretrovirals. This information should be used to counsel women with HIV who desire long-acting reversible contraceptives.
Keywords: Atazanavir; Efavirenz; Etonogestrel; Long-acting reversible contraceptives; Lopinavir; Pharmacokinetics.
Copyright © 2021. Published by Elsevier Inc.
Conflict of interest statement
Conflicts of Interest
The authors report no conflicts of interest related to this work.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Pitts CJ. Update on Clinical Practice Guidelines for Human Immunodeficiency Virus. The Nursing clinics of North America. 2020;55:417–27. - PubMed
-
- UNAIDS. Global HIV & AIDS statistics — 2020 fact sheet. 2020.

