Laminar dynamics of high amplitude beta bursts in human motor cortex
- PMID: 34407440
- PMCID: PMC8463839
- DOI: 10.1016/j.neuroimage.2021.118479
Laminar dynamics of high amplitude beta bursts in human motor cortex
Abstract
Motor cortical activity in the beta frequency range is one of the strongest and most studied movement-related neural signals. At the single trial level, beta band activity is often characterized by transient, high amplitude, bursting events rather than slowly modulating oscillations. The timing of these bursting events is tightly linked to behavior, suggesting a more dynamic functional role for beta activity than previously believed. However, the neural mechanisms underlying beta bursts in sensorimotor circuits are poorly understood. To address this, we here leverage and extend recent developments in high precision MEG for temporally resolved laminar analysis of burst activity, combined with a neocortical circuit model that simulates the biophysical generators of the electrical currents which drive beta bursts. This approach pinpoints the generation of beta bursts in human motor cortex to distinct excitatory synaptic inputs to deep and superficial cortical layers, which drive current flow in opposite directions. These laminar dynamics of beta bursts in motor cortex align with prior invasive animal recordings within the somatosensory cortex, and suggest a conserved mechanism for somatosensory and motor cortical beta bursts. More generally, we demonstrate the ability for uncovering the laminar dynamics of event-related neural signals in human non-invasive recordings. This provides important constraints to theories about the functional role of burst activity for movement control in health and disease, and crucial links between macro-scale phenomena measured in humans and micro-circuit activity recorded from animal models.
Keywords: Beta bursts; High precision MEG; Laminar comparison.
Copyright © 2021. Published by Elsevier Inc.
Figures
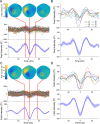


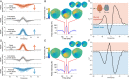

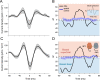
References
-
- Adjamian P., Barnes G.R., Hillebrand A., Holliday I.E., Singh K.D., Furlong P.L., Harrington E., Barclay C.W., Route P.J.G. Co-registration of magnetoencephalography with magnetic resonance imaging using bite-bar-based fiducials and surface-matching. Clin. Neurophysiol. 2004;115:691–698. doi: 10.1016/j.clinph.2003.10.023. - DOI - PubMed
-
- Arikuni T., Watanabe K., Kubota K. Connections of area 8 with area 6 in the brain of the macaque monkey. J. Comp. Neurol. 1988;277:21–40. - PubMed

