Carnitine Orotate Complex Ameliorates Insulin Resistance and Hepatic Steatosis Through Carnitine Acetyltransferase Pathway
- PMID: 34407600
- PMCID: PMC8640142
- DOI: 10.4093/dmj.2020.0223
Carnitine Orotate Complex Ameliorates Insulin Resistance and Hepatic Steatosis Through Carnitine Acetyltransferase Pathway
Abstract
Background: Carnitine orotate complex (Godex) has been shown to decrease glycated hemoglobin levels and improve steatosis in patients with type 2 diabetes mellitus with non-alcoholic fatty liver disease. However, the mechanisms of Godex in glucose metabolism remain unclear.
Methods: Male C57BL/6J mice were divided into four groups: normal-fat diet, high-fat diet, a high-fat diet supplemented with intraperitoneal injection of (500 mg or 2,000 mg/kg/day) Godex for 8 weeks. Computed tomography, indirect calorimetry, and histological analyses including electron microscopy of the liver were performed, and biochemical profiles and oral glucose tolerance test and insulin tolerance test were undertaken. Expressions of genes in the lipid and glucose metabolism, activities of oxidative phosphorylation enzymes, carnitine acetyltransferase, pyruvate dehydrogenase, and acetyl-coenzyme A (CoA)/CoA ratio were evaluated.
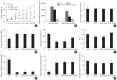
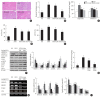
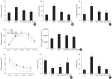
Results: Godex improved insulin sensitivity and significantly decreased fasting plasma glucose, homeostatic model assessment for insulin resistance, steatosis, and gluconeogenesis, with a marked increase in fatty acid oxidation as well as better use of glucose in high-fat diet-fed mice. It preserved mitochondrial function and ultrastructure, restored oxidative phosphorylation enzyme activities, decreased acetyl-CoA/CoA ratio, and increased carnitine acetyltransferase content and pyruvate dehydrogenase activity. Carnitine acetyltransferase knockdown partially reversed the effects of Godex in liver and in vitro.
Conclusion: Godex improved insulin resistance and steatosis by regulating carnitine acetyltransferase in liver in high-fat diet-fed mice.
Keywords: Carnitine O-acetyltransferase; Diet, high-fat; Insulin resistance.
Conflict of interest statement
This study was supported in part by a research grant from Celltrion Pharm Inc., Seoul, Korea. The sponsor had no role in data collection, analysis, interpretation, or writing of the manuscript.
Figures




Comment in
-
The Role of Carnitine Orotate Complex in Fatty Liver.Diabetes Metab J. 2021 Nov;45(6):866-867. doi: 10.4093/dmj.2021.0272. Epub 2021 Nov 22. Diabetes Metab J. 2021. PMID: 34847643 Free PMC article. No abstract available.
References
-
- Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–92. - PubMed
-
- Guo W, Li D, You Y, Li W, Hu B, Zhang S, et al. Cystathionine γ-lyase deficiency aggravates obesity-related insulin resistance-via FoxO1-dependent hepatic gluconeogenesis. FASEB J. 2019;33:4212–24. - PubMed
-
- Park MS, Kang JS, Chon CY, Paik SW, Rim KS, Kwak MJ, et al. Oral Godex capsule for chronic liver disease: a double-blind, randomized, multicenter controlled trial. J Korean Soc Clin Pharmacol Ther. 2001;9:151–62.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

