Construction and preservation of a stable and highly expressed recombinant Helicobacter pylori vacuolating cytotoxin A with apoptotic activity
- PMID: 34407768
- PMCID: PMC8371779
- DOI: 10.1186/s12866-021-02262-7
Construction and preservation of a stable and highly expressed recombinant Helicobacter pylori vacuolating cytotoxin A with apoptotic activity
Abstract
Background: H. pylori is closely related to the occurrence and development of various digestive gastritis, peptic ulcer and mucosa-associated lymphoid tissue (MALT) lymphoma. H. pylori is also a class I carcinogen of gastric cancer. VacA is the only exocrine toxin of H. pylori, which plays a very important role in the pathogenesis of H. pylori. The production of VacA in natural circumstances is complex with heavy workload and low yield. Therefore, it is very important to obtain recombinant VacA protein which is stable and biologically active. This study therefore aims to explore the expression, purification and stable storage of VacA toxin of H. pylori in E.coli, and to provide experimental basis for further exploration of the role of VacA in H. pylori -induced inflammation of cancer.
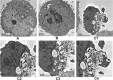
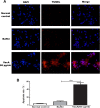
Results: A 2502-bp fragment and VacA gene were identified. An 89.7-kDa VacA34-854 recombinant protein was expressed and purified from the recombinant engineering bacteria and was preserved stably in 50 mM acetic acid buffer (pH 2.9). The amount of the recombinant protein was larger in the inclusion bodies than in the supernatant. In addition, after a 24-h culture with VacA recombinant protein, GES-1 cells demonstrated evidence of apoptosis including early nuclear immobilization and clustering under inverted microscope and TEM. It was found that VacA recombinant protein induced apoptosis by TUNEL assay.
Conclusions: A VacA recombinant protein that is stably and highly expressed and possesses pro-apoptotic activity is successfully constructed. The protein is stably preserved in 50 mM acetic acid buffer (pH 2.9).
Keywords: Apoptosis; Expression; Helicobacter pylori; Preservation; Purification; VacA recombinant protein.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Helicobacter pylori counteracts the apoptotic action of its VacA toxin by injecting the CagA protein into gastric epithelial cells.PLoS Pathog. 2009 Oct;5(10):e1000603. doi: 10.1371/journal.ppat.1000603. Epub 2009 Oct 2. PLoS Pathog. 2009. PMID: 19798427 Free PMC article.
-
Optimized high-purity protein preparation of biologically active recombinant VacA cytotoxin variants from Helicobacter pylori.Protein Expr Purif. 2020 Nov;175:105696. doi: 10.1016/j.pep.2020.105696. Epub 2020 Jul 16. Protein Expr Purif. 2020. PMID: 32681955
-
Induction of gastric epithelial cell apoptosis by Helicobacter pylori vacuolating cytotoxin.Cancer Res. 2003 Mar 1;63(5):951-7. Cancer Res. 2003. PMID: 12615708
-
Vacuolating cytotoxin A (VacA) - A multi-talented pore-forming toxin from Helicobacter pylori.Toxicon. 2016 Aug;118:27-35. doi: 10.1016/j.toxicon.2016.04.037. Epub 2016 Apr 20. Toxicon. 2016. PMID: 27105670 Review.
-
Vacuolating cytotoxin A (VacA), a key toxin for Helicobacter pylori pathogenesis.Front Cell Infect Microbiol. 2012 Jul 12;2:92. doi: 10.3389/fcimb.2012.00092. eCollection 2012. Front Cell Infect Microbiol. 2012. PMID: 22919683 Free PMC article. Review.
Cited by
-
Transient recombinant expression of highly immunogenic CagA, VacA and NapA in Nicotiana benthamiana.Biotechnol Rep (Amst). 2021 Dec 29;33:e00699. doi: 10.1016/j.btre.2021.e00699. eCollection 2022 Mar. Biotechnol Rep (Amst). 2021. PMID: 35028298 Free PMC article.
-
Gastric Epithelial Barrier Disruption, Inflammation and Oncogenic Signal Transduction by Helicobacter pylori.Curr Top Microbiol Immunol. 2023;444:207-238. doi: 10.1007/978-3-031-47331-9_8. Curr Top Microbiol Immunol. 2023. PMID: 38231220 Review.
-
Current study of pathogenetic mechanisms and therapeutics of chronic atrophic gastritis: a comprehensive review.Front Cell Dev Biol. 2024 Dec 10;12:1513426. doi: 10.3389/fcell.2024.1513426. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39720008 Free PMC article. Review.
References
-
- Burucoa C, Axon A. Epidemiology of Helicobacter pylori infection. Helicobacter. 2017;22(Suppl. 1):e12403. 10.1111/hel.12403. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

