Circulating and Intratumoral Adrenal Androgens Correlate with Response to Abiraterone in Men with Castration-Resistant Prostate Cancer
- PMID: 34407973
- PMCID: PMC8563401
- DOI: 10.1158/1078-0432.CCR-21-1819
Circulating and Intratumoral Adrenal Androgens Correlate with Response to Abiraterone in Men with Castration-Resistant Prostate Cancer
Abstract
Purpose: In metastatic castration-resistant prostate cancer (mCRPC) low serum androgens prior to starting abiraterone acetate (AA) is associated with more rapid progression. We evaluated the effect of AA on androgens in castration-resistant prostate cancer (CRPC) metastases and associations of intratumoral androgens with response.
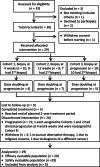
Experimental design: We performed a phase II study of AA plus prednisone in mCRPC. The primary outcome was tissue testosterone at 4 weeks. Exploratory outcomes were association of steroid levels and genomic alterations with response, and escalating AA to 2,000 mg at progression.
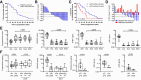
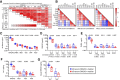
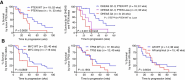
Results: Twenty-nine of 30 men were evaluable. Testosterone in metastatic biopsies became undetectable at 4 weeks (P < 0.001). Serum and tissue dehydroepiandrosterone sulfate (DHEAS) remained detectable in many patients and was not increased at progression. Serum and tissue DHEAS in the lowest quartile (pretreatment), serum DHEAS in the lowest quartile (4 weeks), and undetectable tissue DHEAS (on-therapy) associated with rapid progression (20 vs. 48 weeks, P = 0.0018; 20 vs. 52 weeks, P = 0.0003; 14 vs. 40 weeks, P = 0.0001; 20 vs. 56 weeks, P = 0.02, respectively). One of 16 men escalating to 2,000 mg had a 30% PSA decline; 13 developed radiographic progression by 12 weeks. Among patients with high serum DHEAS at baseline, wild-type (WT) PTEN status associated with longer response (61 vs. 33 weeks, P = 0.02).
Conclusions: Low-circulating adrenal androgen levels are strongly associated with an androgen-poor tumor microenvironment and with poor response to AA. Patients with CRPC with higher serum DHEAS levels may benefit from dual androgen receptor (AR)-pathway inhibition, while those in the lowest quartile may require combinations with non-AR-directed therapy.
©2021 The Authors; Published by the American Association for Cancer Research.
Figures

References
-
- Mohler JL, Gregory CW, Ford OH 3rd, Kim D, Weaver CM, Petrusz P, et al. The androgen axis in recurrent prostate cancer. Clin Cancer Res 2004;10:440–8. - PubMed
-
- Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res 2006;66:2815–25. - PubMed
-
- Taplin ME, Montgomery B, Logothetis CJ, Bubley GJ, Richie JP, Dalkin BL, et al. Intense androgen-deprivation therapy with abiraterone acetate plus leuprolide acetate in patients with localized high-risk prostate cancer: results of a randomized phase II neoadjuvant study. J Clin Oncol 2014;32:3705–15. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

