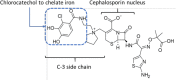
Physicochemical stability of cefiderocol, a novel siderophore cephalosporin, in syringes at 62.5 mg/mL for continuous administration in intensive care units
- PMID: 34407976
- PMCID: PMC10086710
- DOI: 10.1136/ejhpharm-2021-002935
Physicochemical stability of cefiderocol, a novel siderophore cephalosporin, in syringes at 62.5 mg/mL for continuous administration in intensive care units
Abstract
Introduction: Cefiderocol is a new siderophore time-dependent antibiotic of last resort. The manufacturer reports a stability of 6 hours for the infusion solution diluted in normal saline (NS) or dextrose 5% in water (D5W) for a concentration between 7.5 and 20 mg/mL. Optimising its effectiveness by continuous infusion is crucial. The aim of this work was to study the physicochemical stability of cefiderocol diluted in NS or D5W in polypropylene syringes for 48 hours at a concentration of 62.5 mg/mL stored at room temperature, protected or not from light.
Materials and methods: Three preparations for each condition were performed. At each time of the analysis, one sample for each preparation was analysed in triplicate by a validated high performance liquid chromatography method coupled to a photodiode array detector at 260 nm. Particle contamination, absorbance measurement, visual inspection and pH measurement were assessed. The limit of stability was set at 90% of the initial concentration, without physical modification.
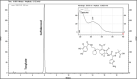
Results: The linearity was validated with an R² of 0.9999. The coefficients of variation for repeatability and intermediate precision were less than 2%. In NS and D5W, cefiderocol retained more than 90% of the initial concentration after 12 hours in syringes, exposed or not to light. Two degradation products (nos 2 and 11, observed during forced degradation) were detected during the stability study. The absorbance at 410 nm increased progressively, regardless of the storage conditions. The particulate contamination test met the specifications of the container. pH values were all between 5.22 and 5.32. No visual changes were detected.
Conclusion: In polypropylene syringes, cefiderocol 62.5 mg/mL (3 g in 48 mL) diluted in NS or D5W was stable for 12 hours at room temperature. These new data allow the use of cefiderocol in continuous infusion.
Keywords: administration; critical care; intravenous; microbiology; pharmaceutical preparations.
© European Association of Hospital Pharmacists 2023. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Fetcroja® . European Medicines Agency, 2020. https://www.ema.europa.eu/en/medicines/human/EPAR/fetcroja
-
- Bassetti M, Echols R, Matsunaga Y, et al. . Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant gram-negative bacteria (CREDIBLE-CR): a randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect Dis 2021;21:226–40. 10.1016/S1473-3099(20)30796-9 - DOI - PubMed
-
- Fetcroja® 1 g powder - Summary of Product Characteristics (SmPC) - (EMC). Available: https://www.medicines.org.uk/emc/product/11771#gref
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous