Combined use of a broad-panel respiratory multiplex PCR and procalcitonin to reduce duration of antibiotics exposure in patients with severe community-acquired pneumonia (MULTI-CAP): a multicentre, parallel-group, open-label, individual randomised trial conducted in French intensive care units
- PMID: 34408046
- PMCID: PMC8375718
- DOI: 10.1136/bmjopen-2020-048187
Combined use of a broad-panel respiratory multiplex PCR and procalcitonin to reduce duration of antibiotics exposure in patients with severe community-acquired pneumonia (MULTI-CAP): a multicentre, parallel-group, open-label, individual randomised trial conducted in French intensive care units
Abstract
Introduction: At the time of the worrying emergence and spread of bacterial resistance, reducing the selection pressure by reducing the exposure to antibiotics in patients with community-acquired pneumonia (CAP) is a public health issue. In this context, the combined use of molecular tests and biomarkers for guiding antibiotics discontinuation is attractive. Therefore, we have designed a trial comparing an integrated approach of diagnosis and treatment of severe CAP to usual care.
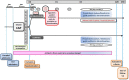
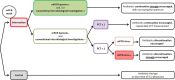
Methods and analysis: The multiplex PCR and procalcitonin to reduce duration of antibiotics exposure in patients with severe-CAP (MULTI-CAP) trial is a multicentre (n=20), parallel-group, superiority, open-label, randomised trial. Patients are included if adult admitted to intensive care unit for a CAP. Diagnosis of pneumonia is based on clinical criteria and a newly appeared parenchymal infiltrate. Immunocompromised patients are excluded. Subjects are randomised (1:1 ratio) to either the intervention arm (experimental strategy) or the control arm (usual strategy). In the intervention arm, the microbiological diagnosis combines a respiratory multiplex PCR (mPCR) and conventional microbiological investigations. An algorithm of early antibiotic de-escalation or discontinuation is recommended, based on mPCR results and the procalcitonin value. In the control arm, only conventional microbiological investigations are performed and antibiotics de-escalation remains at the clinician's discretion. The primary endpoint is the number of days alive without any antibiotic from the randomisation to day 28. Based on our hypothesis of 2 days gain in the intervention arm, we aim to enrol a total of 450 patients over a 30-month period.
Ethics and dissemination: The MULTI-CAP trial is conducted according to the principles of the Declaration of Helsinki, is registered in Clinical Trials and has been approved by the Committee for Protection of Persons and the National French Drug Safety Agency. Written informed consents are obtained from all the patients (or representatives). The results will be disseminated through educational institutions, submitted to peer-reviewed journals for publication and presented at medical congresses.
Trial registration number: NCT03452826; Pre-results.
Keywords: adult intensive & critical care; adult thoracic medicine; diagnostic microbiology; molecular diagnostics; respiratory infections.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Fine MJ, Hough LJ, Medsger AR, et al. . The hospital admission decision for patients with community-acquired pneumonia. Results from the pneumonia patient outcomes research team cohort study. Arch Intern Med 1997;157:36–44. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
