Improving uptake of Fracture Prevention drug treatments: a protocol for Development of a consultation intervention (iFraP-D)
- PMID: 34408051
- PMCID: PMC8375717
- DOI: 10.1136/bmjopen-2021-048811
Improving uptake of Fracture Prevention drug treatments: a protocol for Development of a consultation intervention (iFraP-D)
Abstract
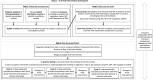
Introduction: Prevention of fragility fractures, a source of significant economic and personal burden, is hindered by poor uptake of fracture prevention medicines. Enhancing communication of scientific evidence and elicitation of patient medication-related beliefs has the potential to increase patient commitment to treatment. The Improving uptake of Fracture Prevention drug treatments (iFraP) programme aims to develop and evaluate a theoretically informed, complex intervention consisting of a computerised web-based decision support tool, training package and information resources, to facilitate informed decision-making about fracture prevention treatment, with a long-term aim of improving informed treatment adherence. This protocol focuses on the iFraP Development (iFraP-D) work.
Methods and analysis: The approach to iFraP-D is informed by the Medical Research Council complex intervention development and evaluation framework and the three-step implementation of change model. The context for the study is UK fracture liaison services (FLS), which enact secondary fracture prevention. An evidence synthesis of clinical guidelines and Delphi exercise will be conducted to identify content for the intervention. Focus groups with patients, FLS clinicians and general practitioners and a usual care survey will facilitate understanding of current practice, and investigate barriers and facilitators to change. Design of the iFraP intervention will be informed by decision aid development standards and theories of implementation, behaviour change, acceptability and medicines adherence. The principles of co-design will underpin all elements of the study through a dedicated iFraP community of practice including key stakeholders and patient advisory groups. In-practice testing of the prototype intervention will inform revisions ready for further testing in a subsequent pilot and feasibility randomised trial.
Ethics and dissemination: Ethical approval was obtained from North West-Greater Manchester West Research Ethics Committee (19/NW/0559). Dissemination and knowledge mobilisation will be facilitated through national bodies and networks, publications and presentations.
Trial registration number: researchregistry5041.
Keywords: musculoskeletal disorders; qualitative research; rheumatology.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: ZP reports grants from the NIHR Clinician Scientist Award (CS-2018-18-ST2-010), Royal Osteoporosis Society and the Haywood Rheumatology Research and Development Foundation to conduct this study. CJ reports funding from the NIHR Applied Research Collaboration (ARC) West Midlands; CM reports grants from the NIHR Research Professorship in General Practice (NIHR-RP-2014-04-026), the NIHR School for Primary Care Research and NIHR Applied Research Collaborations (West Midlands), Wellcome, Medical Research Council, Dunhill, Versus Arthritis and Bristol Myer-Squibb, outside the submitted work; FC-M reports grants from NIHR Clinical Research Network Scholar Programme; ST and SRC report receiving funds from National Institute for Health Research (NIHR) (Clinician Scientist Award (CS-2018-18-ST2-010)/NIHR Academy) and the Royal Osteoporosis Society via Prescribing Decision Support Ltd to support the development of the iFraP decision support tool; TWO’N reports grants and non-financial support from AMGEN and the NIHR Manchester Biomedical Research Centre outside the submitted work; CI reports grants from NIHR during the conduct of the study and disclosed being a member of NICE’s Medical Technologies Advisory Committee outside the submitted work; JJE reports grants as a NIHR Academic Clinical Lecturer in Primary Care (CL-2016-10-003). ECo reports grants from Versus Arthritis, NIHR RfPB, HQIP and the Royal Osteoporosis Society.
Figures

Similar articles
-
Exploring practice and perspectives on shared decision-making about osteoporosis medicines in Fracture Liaison Services: the iFraP development qualitative study.Arch Osteoporos. 2024 Jun 19;19(1):50. doi: 10.1007/s11657-024-01410-6. Arch Osteoporos. 2024. PMID: 38898212 Free PMC article.
-
Developing a model Fracture Liaison Service consultation with patients, carers and clinicians: a Delphi survey to inform content of the iFraP complex consultation intervention.Arch Osteoporos. 2021 Mar 24;16(1):58. doi: 10.1007/s11657-021-00913-w. Arch Osteoporos. 2021. PMID: 33761007 Free PMC article.
-
The Michael Mason Prize: Development and feasibility testing of a complex intervention to improve adherence to fracture prevention medicine.Rheumatology (Oxford). 2025 Aug 20:keaf413. doi: 10.1093/rheumatology/keaf413. Online ahead of print. Rheumatology (Oxford). 2025. PMID: 40833931
-
Secondary prevention of fragility fractures in Asia Pacific: an educational initiative.Osteoporos Int. 2020 May;31(5):805-826. doi: 10.1007/s00198-019-05197-y. Epub 2019 Dec 1. Osteoporos Int. 2020. PMID: 31788717 Review.
-
Fracture liaison services improve outcomes of patients with osteoporosis-related fractures: A systematic literature review and meta-analysis.Bone. 2018 Jun;111:92-100. doi: 10.1016/j.bone.2018.03.018. Epub 2018 Mar 16. Bone. 2018. PMID: 29555309
Cited by
-
Research priorities regarding the use of bisphosphonates for osteoporosis: a UK priority setting exercise.Osteoporos Int. 2023 Oct;34(10):1711-1718. doi: 10.1007/s00198-023-06806-7. Epub 2023 Jun 9. Osteoporos Int. 2023. PMID: 37294333
-
Variation in UK fracture liaison service consultation conduct and content before and during the COVID pandemic: results from the iFraP-D UK survey.Arch Osteoporos. 2023 Dec 20;19(1):5. doi: 10.1007/s11657-023-01361-4. Arch Osteoporos. 2023. PMID: 38123745 Free PMC article.
-
Exploring practice and perspectives on shared decision-making about osteoporosis medicines in Fracture Liaison Services: the iFraP development qualitative study.Arch Osteoporos. 2024 Jun 19;19(1):50. doi: 10.1007/s11657-024-01410-6. Arch Osteoporos. 2024. PMID: 38898212 Free PMC article.
-
A person-centred primary care pharmacist-led osteoporosis review for optimising medicines (PHORM): a protocol for the development and co-design of a model consultation intervention.BMJ Open. 2024 Nov 2;14(11):e085323. doi: 10.1136/bmjopen-2024-085323. BMJ Open. 2024. PMID: 39488418 Free PMC article.
-
Acceptability of, and preferences for, remote consulting during COVID-19 among older patients with two common long-term musculoskeletal conditions: findings from three qualitative studies and recommendations for practice.BMC Musculoskelet Disord. 2022 Apr 2;23(1):312. doi: 10.1186/s12891-022-05273-1. BMC Musculoskelet Disord. 2022. PMID: 35366845 Free PMC article.
References
-
- National Osteoporosis Society . The osteoporosis agenda England: improving the lives of people with osteoporosis and fragility fractures, 2015. Available: https://theros.org.uk/media/1959/agenda-for-osteoporosis-england-final.pdf
-
- NHS RightCare . Nhs RightCare scenario: the variation between sub-optimal and optimal pathways, 2017. Available: https://www.england.nhs.uk/rightcare/wp-content/uploads/sites/40/2017/02...
-
- National Osteoporosis Society . Effective secondary prevention of fragility fractures: clinical standards for fracture liaison services, 2015. Available: https://theros.org.uk/media/2082/clinical-standards-for-fracture-liaison...
-
- National Institute for Health and Care Excellence . Bisphosphonates for treating osteoporosis, 2017. Available: https://www.nice.org.uk/guidance/ta464 [Accessed 21 Jan 2020].
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
