Status, use and impact of sharing individual participant data from clinical trials: a scoping review
- PMID: 34408052
- PMCID: PMC8375721
- DOI: 10.1136/bmjopen-2021-049228
Status, use and impact of sharing individual participant data from clinical trials: a scoping review
Abstract
Objectives: To explore the impact of data-sharing initiatives on the intent to share data, on actual data sharing, on the use of shared data and on research output and impact of shared data.
Eligibility criteria: All studies investigating data-sharing practices for individual participant data (IPD) from clinical trials.
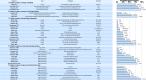
Sources of evidence: We searched the Medline database, the Cochrane Library, the Science Citation Index Expanded and the Social Sciences Citation Index via Web of Science, and preprints and proceedings of the International Congress on Peer Review and Scientific Publication. In addition, we inspected major clinical trial data-sharing platforms, contacted major journals/publishers, editorial groups and some funders.
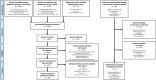
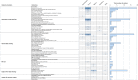
Charting methods: Two reviewers independently extracted information on methods and results from resources identified using a standardised questionnaire. A map of the extracted data was constructed and accompanied by a narrative summary for each outcome domain.
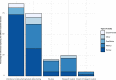
Results: 93 studies identified in the literature search (published between 2001 and 2020, median: 2018) and 5 from additional information sources were included in the scoping review. Most studies were descriptive and focused on early phases of the data-sharing process. While the willingness to share IPD from clinical trials is extremely high, actual data-sharing rates are suboptimal. A survey of journal data suggests poor to moderate enforcement of the policies by publishers. Metrics provided by platforms suggest that a large majority of data remains unrequested. When requested, the purpose of the reuse is more often secondary analyses and meta-analyses, rarely re-analyses. Finally, studies focused on the real impact of data-sharing were rare and used surrogates such as citation metrics.
Conclusions: There is currently a gap in the evidence base for the impact of IPD sharing, which entails uncertainties in the implementation of current data-sharing policies. High level evidence is needed to assess whether the value of medical research increases with data-sharing practices.
Keywords: health informatics; information management; information technology.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

Similar articles
-
Beyond the black stump: rapid reviews of health research issues affecting regional, rural and remote Australia.Med J Aust. 2020 Dec;213 Suppl 11:S3-S32.e1. doi: 10.5694/mja2.50881. Med J Aust. 2020. PMID: 33314144
-
Obtaining and managing data sets for individual participant data meta-analysis: scoping review and practical guide.BMC Med Res Methodol. 2020 May 12;20(1):113. doi: 10.1186/s12874-020-00964-6. BMC Med Res Methodol. 2020. PMID: 32398016 Free PMC article.
-
Evaluation of Data Sharing After Implementation of the International Committee of Medical Journal Editors Data Sharing Statement Requirement.JAMA Netw Open. 2021 Jan 4;4(1):e2033972. doi: 10.1001/jamanetworkopen.2020.33972. JAMA Netw Open. 2021. PMID: 33507256 Free PMC article.
-
Prevalence and predictors of data and code sharing in the medical and health sciences: systematic review with meta-analysis of individual participant data.BMJ. 2023 Jul 11;382:e075767. doi: 10.1136/bmj-2023-075767. BMJ. 2023. PMID: 37433624 Free PMC article.
-
Updating insights into rosiglitazone and cardiovascular risk through shared data: individual patient and summary level meta-analyses.BMJ. 2020 Feb 5;368:l7078. doi: 10.1136/bmj.l7078. BMJ. 2020. PMID: 32024657 Free PMC article.
Cited by
-
How do we measure the costs, benefits, and harms of sharing data from biomedical studies? A protocol for a scoping review.Open Res Eur. 2025 Jan 14;3:151. doi: 10.12688/openreseurope.16063.2. eCollection 2023. Open Res Eur. 2025. PMID: 39867822 Free PMC article.
-
Open video data sharing in developmental science and clinical practice.iScience. 2023 Mar 7;26(4):106348. doi: 10.1016/j.isci.2023.106348. eCollection 2023 Apr 21. iScience. 2023. PMID: 36994082 Free PMC article.
-
Compliance with International Committee of Medical Journal Editors policy on individual participant data sharing in clinical trial registries: An audit.Perspect Clin Res. 2022 Oct-Dec;13(4):213-214. doi: 10.4103/picr.picr_85_22. Epub 2022 Sep 12. Perspect Clin Res. 2022. PMID: 36337374 Free PMC article. No abstract available.
-
Establishing a Pregnancy Lyme Disease Biobank.Methods Mol Biol. 2024;2742:245-257. doi: 10.1007/978-1-0716-3561-2_17. Methods Mol Biol. 2024. PMID: 38165627
-
Data-sharing recommendations in biomedical journals and randomised controlled trials: an audit of journals following the ICMJE recommendations.BMJ Open. 2020 May 30;10(5):e038887. doi: 10.1136/bmjopen-2020-038887. BMJ Open. 2020. PMID: 32474433 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
