Modality-Specific Modulation of Temperature Representations in the Spinal Cord after Injury
- PMID: 34408066
- PMCID: PMC8482863
- DOI: 10.1523/JNEUROSCI.1104-21.2021
Modality-Specific Modulation of Temperature Representations in the Spinal Cord after Injury
Abstract
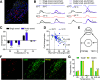
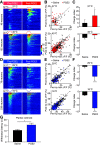
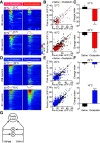
Different types of tissue injury, such as inflammatory and neuropathic conditions, cause modality-specific alternations on temperature perception. There are profound changes in peripheral sensory neurons after injury, but how patterned neuronal activities in the CNS encode injury-induced sensitization to temperature stimuli is largely unknown. Using in vivo calcium imaging and mouse genetics, we show that formalin- and prostaglandin E2-induced inflammation dramatically increase spinal responses to heating and decrease responses to cooling in male and female mice. The reduction of cold response is largely eliminated on ablation of TRPV1-expressing primary sensory neurons, indicating a crossover inhibition of cold response from the hyperactive heat inputs in the spinal cord. Interestingly, chemotherapy medication oxaliplatin can rapidly increase spinal responses to cooling and suppress responses to heating. Together, our results suggest a push-pull mechanism in processing cold and heat inputs and reveal a synergic mechanism to shift thermosensation after injury.SIGNIFICANCE STATEMENT In this paper, we combine our novel in vivo spinal cord two-photon calcium imaging, mouse genetics, and persistent pain models to study how tissue injury alters the sensation of temperature. We discover modality-specific changes of spinal temperature responses in different models of injury. Chemotherapy medication oxaliplatin leads to cold hypersensitivity and heat hyposensitivity. By contrast, inflammation increases heat sensitivity and decreases cold sensitivity. This decrease in cold sensitivity results from the stronger crossover inhibition from the hyperactive heat inputs. Our work reveals the bidirectional change of thermosensitivity by injury and suggests that the crossover inhibitory circuit underlies the shifted thermosensation, providing a mechanism to the biased perception toward a unique thermal modality that was observed clinically in chronic pain patients.
Keywords: crossover inhibition; in vivo calcium imaging; injury; spinal cord; thermosensation.
Copyright © 2021 the authors.
Figures


Similar articles
-
The cellular code for mammalian thermosensation.J Neurosci. 2013 Mar 27;33(13):5533-41. doi: 10.1523/JNEUROSCI.5788-12.2013. J Neurosci. 2013. PMID: 23536068 Free PMC article.
-
Sensory determinants of thermal pain.Brain. 2002 Mar;125(Pt 3):501-10. doi: 10.1093/brain/awf055. Brain. 2002. PMID: 11872608
-
Optogenetic Inhibition of CGRPα Sensory Neurons Reveals Their Distinct Roles in Neuropathic and Incisional Pain.J Neurosci. 2018 Jun 20;38(25):5807-5825. doi: 10.1523/JNEUROSCI.3565-17.2018. J Neurosci. 2018. PMID: 29925650 Free PMC article.
-
How is chronic pain related to sympathetic dysfunction and autonomic dysreflexia following spinal cord injury?Auton Neurosci. 2018 Jan;209:79-89. doi: 10.1016/j.autneu.2017.01.006. Epub 2017 Jan 27. Auton Neurosci. 2018. PMID: 28161248 Free PMC article. Review.
-
Probing the coding logic of thermosensation using spinal cord calcium imaging.Exp Neurol. 2019 Aug;318:42-49. doi: 10.1016/j.expneurol.2019.04.009. Epub 2019 Apr 20. Exp Neurol. 2019. PMID: 31014574 Free PMC article. Review.
Cited by
-
Excitatory and Inhibitory Neurons of the Spinal Cord Superficial Dorsal Horn Diverge in Their Somatosensory Responses and Plasticity in Vivo.J Neurosci. 2022 Mar 9;42(10):1958-1973. doi: 10.1523/JNEUROSCI.1860-21.2021. Epub 2022 Jan 19. J Neurosci. 2022. PMID: 35046121 Free PMC article.
-
TiO2-Nanowired Delivery of Chinese Extract of Ginkgo biloba EGb-761 and Bilobalide BN-52021 Enhanced Neuroprotective Effects of Cerebrolysin Following Spinal Cord Injury at Cold Environment.Adv Neurobiol. 2023;32:353-384. doi: 10.1007/978-3-031-32997-5_9. Adv Neurobiol. 2023. PMID: 37480466 Review.
-
Nociception and pain in humans lacking a functional TRPV1 channel.J Clin Invest. 2023 Feb 1;133(3):e153558. doi: 10.1172/JCI153558. J Clin Invest. 2023. PMID: 36454632 Free PMC article.
-
Differential modification of ascending spinal outputs in acute and chronic pain states.Neuron. 2025 Apr 16;113(8):1223-1239.e5. doi: 10.1016/j.neuron.2025.01.031. Epub 2025 Feb 28. Neuron. 2025. PMID: 40023166
-
Cell type-specific calcium imaging of central sensitization in mouse dorsal horn.Nat Commun. 2022 Sep 3;13(1):5199. doi: 10.1038/s41467-022-32608-2. Nat Commun. 2022. PMID: 36057681 Free PMC article.
References
-
- Abraira VE, Kuehn ED, Chirila AM, Springel MW, Toliver AA, Zimmerman AL, Orefice LL, Boyle KA, Bai L, Song BJ, Bashista KA, O'Neill TG, Zhuo J, Tsan C, Hoynoski J, Rutlin M, Kus L, Niederkofler V, Watanabe M, Dymecki SM, et al. . (2017) The cellular and synaptic architecture of the mechanosensory dorsal horn. Cell 168:295–310.e219. 10.1016/j.cell.2016.12.010 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
