Blocking α4β7 integrin delays viral rebound in SHIVSF162P3-infected macaques treated with anti-HIV broadly neutralizing antibodies
- PMID: 34408080
- PMCID: PMC8977869
- DOI: 10.1126/scitranslmed.abf7201
Blocking α4β7 integrin delays viral rebound in SHIVSF162P3-infected macaques treated with anti-HIV broadly neutralizing antibodies
Abstract
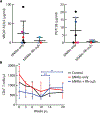
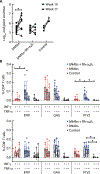
Anti-HIV broadly neutralizing antibodies (bNAbs) may favor development of antiviral immunity by engaging the immune system during immunotherapy. Targeting integrin α4β7 with an anti-α4β7 monoclonal antibody (Rh-α4β7) affects immune responses in SIV/SHIV-infected macaques. To explore the therapeutic potential of combining bNAbs with α4β7 integrin blockade, SHIVSF162P3-infected, viremic rhesus macaques were treated with bNAbs only (VRC07-523LS and PGT128 anti-HIV antibodies) or a combination of bNAbs and Rh-α4β7 or were left untreated as a control. Treatment with bNAbs alone decreased viremia below 200 copies/ml in all macaques, but seven of eight macaques (87.5%) in the bNAbs-only group rebounded within a median of 3 weeks (95% CI: 2 to 9). In contrast, three of six macaques treated with a combination of Rh-α4β7 and bNAbs (50%) maintained a viremia below 200 copies/ml until the end of the follow-up period; viremia in the other three macaques rebounded within a median of 6 weeks (95% CI: 5 to 11). Thus, there was a modest delay in viral rebound in the macaques treated with the combination antibody therapy compared to bNAbs alone. Our study suggests that α4β7 integrin blockade may prolong virologic control by bNAbs in SHIVSF162P3-infected macaques.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures




References
-
- Barouch DH, Whitney JB, Moldt B, Klein F, Oliveira TY, Liu J, Stephenson KE, Chang HW, Shekhar K, Gupta S, Nkolola JP, Seaman MS, Smith KM, Borducchi EN, Cabral C, Smith JY, Blackmore S, Sanisetty S, Perry JR, Beck M, Lewis MG, Rinaldi W, Chakraborty AK, Poignard P, Nussenzweig MC, Burton DR, Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature 503, 224–228 (2013). - PMC - PubMed
-
- Julg B, Pegu A, Abbink P, Liu J, Brinkman A, Molloy K, Mojta S, Chandrashekar A, Callow K, Wang K, Chen X, Schmidt SD, Huang J, Koup RA, Seaman MS, Keele BF, Mascola JR, Connors M, Barouch DH, Virological control by the CD4-binding site antibody N6 in simian-HIV-infected rhesus monkeys. J. Virol. 91, e00498–17 (2017). - PMC - PubMed
-
- Caskey M, Klein F, Lorenzi JC, Seaman MS, West AP Jr., Buckley N, Kremer G, Nogueira L, Braunschweig M, Scheid JF, Horwitz JA, Shimeliovich I, Ben-Avraham S, Witmer-Pack M, Platten M, Lehmann C, Burke LA, Hawthorne T, Gorelick RJ, Walker BD, Keler T, Gulick RM, Fatkenheuer G, Schlesinger SJ, Nussenzweig MC, Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature 522, 487–491 (2015). - PMC - PubMed
-
- Caskey M, Schoofs T, Gruell H, Settler A, Karagounis T, Kreider EF, Murrell B, Pfeifer N, Nogueira L, Oliveira TY, Learn GH, Cohen YZ, Lehmann C, Gillor D, Shimeliovich I, Unson-O’Brien C, Weiland D, Robles A, Kummerle T, Wyen C, Levin R, Witmer-Pack M, Eren K, Ignacio C, Kiss S, West AP Jr., Mouquet H, Zingman BS, Gulick RM, Keler T, Bjorkman PJ, Seaman MS, Hahn BH, Fatkenheuer G, Schlesinger SJ, Nussenzweig MC, Klein F, Antibody 10–1074 suppresses viremia in HIV-1-infected individuals. Nat. Med. 23, 185–191 (2017). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

