Development of a monoclonal antibody for the detection of anti-canine CD20 chimeric antigen receptor expression on canine CD20 chimeric antigen receptor-transduced T cells
- PMID: 34408098
- PMCID: PMC8569873
- DOI: 10.1292/jvms.21-0326
Development of a monoclonal antibody for the detection of anti-canine CD20 chimeric antigen receptor expression on canine CD20 chimeric antigen receptor-transduced T cells
Abstract
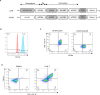
Chimeric antigen receptor (CAR) CAR-T cell therapy targeting CD20 can be a novel adoptive cell therapy for canine patients with B-cell malignancy. After injection of the CAR-T cells in vivo, monitoring circulating CAR-T cells is essential to prove in vivo persistence of CAR-T cells. In this study, we developed a novel monoclonal antibody against canine CD20 CAR, whose single-chain variable fragment was derived from the our previously reported anti-canine CD20 therapeutic antibody. Furthermore, we proved that this monoclonal antibody can detect therapeutic anti-canine CD20 chimeric antibody in the serum from healthy beagle dogs injected with the therapeutic antibody for safety study. This monoclonal antibody is a useful tool for monitoring both canine CD20-CAR-T cells and anti-canine CD20 therapeutic antibody for canine lymphoma.
Keywords: CD20; adoptive immunotherapy; canine; chimeric antigen receptor T cell; monoclonal antibody.
Figures

Similar articles
-
Optimization of canine CD20 chimeric antigen receptor T cell manufacturing and in vitro cytotoxic activity against B-cell lymphoma.Vet Comp Oncol. 2020 Dec;18(4):739-752. doi: 10.1111/vco.12602. Epub 2020 Jun 1. Vet Comp Oncol. 2020. PMID: 32329214
-
Optimization of Culture Conditions for the Generation of Canine CD20-CAR-T Cells for Adoptive Immunotherapy.In Vivo. 2022 Mar-Apr;36(2):764-772. doi: 10.21873/invivo.12763. In Vivo. 2022. PMID: 35241532 Free PMC article.
-
Feasibility and Safety of RNA-transfected CD20-specific Chimeric Antigen Receptor T Cells in Dogs with Spontaneous B Cell Lymphoma.Mol Ther. 2016 Sep;24(9):1602-14. doi: 10.1038/mt.2016.146. Epub 2016 Jul 12. Mol Ther. 2016. PMID: 27401141 Free PMC article.
-
Clinical development of anti-CD19 chimeric antigen receptor T-cell therapy for B-cell non-Hodgkin lymphoma.Cancer Sci. 2017 Jun;108(6):1109-1118. doi: 10.1111/cas.13239. Epub 2017 May 25. Cancer Sci. 2017. PMID: 28301076 Free PMC article. Review.
-
Chimeric antigen receptor T-cell therapy for the treatment of aggressive B-cell non-Hodgkin lymphomas: efficacy, toxicity, and comparative chimeric antigen receptor products.Expert Opin Biol Ther. 2019 Nov;19(11):1157-1164. doi: 10.1080/14712598.2019.1644316. Epub 2019 Jul 25. Expert Opin Biol Ther. 2019. PMID: 31342797 Review.
Cited by
-
Development of anti-feline PD-1 antibody and its functional analysis.Sci Rep. 2023 Apr 24;13(1):6420. doi: 10.1038/s41598-023-31543-6. Sci Rep. 2023. PMID: 37095139 Free PMC article.
References
-
- Berinstein N. L., Grillo-López A. J., White C. A., Bence-Bruckler I., Maloney D., Czuczman M., Green D., Rosenberg J., McLaughlin P., Shen D.1998. Association of serum Rituximab (IDEC-C2B8) concentration and anti-tumor response in the treatment of recurrent low-grade or follicular non-Hodgkin’s lymphoma. Ann. Oncol. 9: 995–1001. doi: 10.1023/A:1008416911099 - DOI - PubMed
-
- Guedan S., Posey A. D., Jr, Shaw C., Wing A., Da T., Patel P. R., McGettigan S. E., Casado-Medrano V., Kawalekar O. U., Uribe-Herranz M., Song D., Melenhorst J. J., Lacey S. F., Scholler J., Keith B., Young R. M., June C. H.2018. Enhancing CAR T cell persistence through ICOS and 4-1BB costimulation. JCI Insight 3: e96976. doi: 10.1172/jci.insight.96976 - DOI - PMC - PubMed

