Longitudinal analysis of antibody decay in convalescent COVID-19 patients
- PMID: 34408200
- PMCID: PMC8373894
- DOI: 10.1038/s41598-021-96171-4
Longitudinal analysis of antibody decay in convalescent COVID-19 patients
Abstract
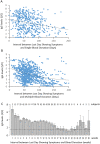
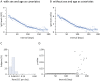
Determining the sustainability of antibodies targeting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is essential for predicting immune response against the Coronavirus disease 2019 (COVID-19). To quantify the antibody decay rates among the varying levels of anti-nucleocapsid (anti-N) Immunoglobulin G (IgG) in convalescent COVID-19 patients and estimate the length of time they maintained SARS-CoV-2 specific antibodies, we have collected longitudinal blood samples from 943 patients over the course of seven months after their initial detection of SARS-CoV-2 virus by RT-PCR. Anti-N IgG levels were then quantified in these blood samples. The primary study outcome was the comparison of antibody decay rates from convalescent patients with high or low initial levels of antibodies using a mixed linear model. Additional measures include the length of time that patients maintain sustainable levels of anti-N IgG. Antibody quantification of blood samples donated by the same subject multiple times shows a gradual decrease of IgG levels to the cutoff index level of 1.4 signal/cut-off (S/C) on the Abbott Architect SARS-CoV-2 IgG test. In addition, this study shows that antibody reduction rate is dependent on initial IgG levels, and patients with initial IgG levels above 3 S/C show a significant 1.68-fold faster reduction rate compared to those with initial IgG levels below 3 S/C. For a majority of the donors naturally occurring anti-N antibodies were detected above the threshold for only four months after infection with SARS-CoV-2. This study is clinically important for the prediction of immune response capacity in COVID-19 patients.
© 2021. The Author(s).
Conflict of interest statement
GRC is an employee of MRN Diagnostics and associates with Mayo Clinic Expanded Access Program (EAP) for collection of convalescent plasma through their nonprofit blood bank Innovative Transfusion Medicine. Other authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

