The endosomal Q-SNARE, Syntaxin 7, defines a rapidly replenishing synaptic vesicle recycling pool in hippocampal neurons
- PMID: 34408265
- PMCID: PMC8373932
- DOI: 10.1038/s42003-021-02512-4
The endosomal Q-SNARE, Syntaxin 7, defines a rapidly replenishing synaptic vesicle recycling pool in hippocampal neurons
Abstract
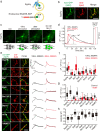
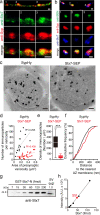
Upon the arrival of repetitive stimulation at the presynaptic terminals of neurons, replenishment of readily releasable synaptic vesicles (SVs) with vesicles in the recycling pool is important for sustained neurotransmitter release. Kinetics of replenishment and the available pool size define synaptic performance. However, whether all SVs in the recycling pool are recruited for release with equal probability and speed is unknown. Here, based on comprehensive optical imaging of various presynaptic endosomal SNARE proteins in cultured hippocampal neurons, all of which are implicated in organellar membrane fusion in non-neuronal cells, we show that part of the recycling pool bearing the endosomal Q-SNARE, syntaxin 7 (Stx7), is preferentially mobilized for release during high-frequency repetitive stimulation. Recruitment of the SV pool marked with an Stx7-reporter requires actin polymerization, as well as activation of the Ca2+/calmodulin signaling pathway, reminiscent of rapidly replenishing SVs characterized previously in calyx of Held synapses. Furthermore, disruption of Stx7 function by overexpressing its N-terminal domain selectively abolished this pool. Thus, our data indicate that endosomal membrane fusion involving Stx7 forms rapidly replenishing vesicles essential for synaptic responses to high-frequency repetitive stimulation, and also highlight functional diversities of endosomal SNAREs in generating distinct exocytic vesicles in the presynaptic terminals.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Dynamic Partitioning of Synaptic Vesicle Pools by the SNARE-Binding Protein Tomosyn.J Neurosci. 2016 Nov 2;36(44):11208-11222. doi: 10.1523/JNEUROSCI.1297-16.2016. J Neurosci. 2016. PMID: 27807164 Free PMC article.
-
Presynaptic loss of dynamin-related protein 1 impairs synaptic vesicle release and recycling at the mouse calyx of Held.J Physiol. 2018 Dec;596(24):6263-6287. doi: 10.1113/JP276424. Epub 2018 Nov 10. J Physiol. 2018. PMID: 30285293 Free PMC article.
-
VAMP4 Maintains a Ca2+-Sensitive Pool of Spontaneously Recycling Synaptic Vesicles.J Neurosci. 2020 Jul 8;40(28):5389-5401. doi: 10.1523/JNEUROSCI.2386-19.2020. Epub 2020 Jun 12. J Neurosci. 2020. PMID: 32532887 Free PMC article.
-
Synaptic Vesicle Endocytosis and Endosomal Recycling in Central Nerve Terminals: Discrete Trafficking Routes?Neuroscientist. 2015 Aug;21(4):413-23. doi: 10.1177/1073858414542251. Epub 2014 Jul 15. Neuroscientist. 2015. PMID: 25027635 Review.
-
Kinetics of transmitter release at the calyx of Held synapse.Proc Jpn Acad Ser B Phys Biol Sci. 2018;94(3):139-152. doi: 10.2183/pjab.94.010. Proc Jpn Acad Ser B Phys Biol Sci. 2018. PMID: 29526973 Free PMC article. Review.
Cited by
-
Transmembrane Batten Disease Proteins Interact With a Shared Network of Vesicle Sorting Proteins, Impacting Their Synaptic Enrichment.Front Neurosci. 2022 May 25;16:834780. doi: 10.3389/fnins.2022.834780. eCollection 2022. Front Neurosci. 2022. PMID: 35692423 Free PMC article.
-
Adaptor protein AP-3 produces synaptic vesicles that release at high frequency by recruiting phospholipid flippase ATP8A1.Nat Neurosci. 2023 Oct;26(10):1685-1700. doi: 10.1038/s41593-023-01434-0. Epub 2023 Sep 18. Nat Neurosci. 2023. PMID: 37723322
-
Live-cell imaging of endocytosed synaptophysin around individual hippocampal presynaptic active zones.Front Cell Neurosci. 2023 Oct 19;17:1277729. doi: 10.3389/fncel.2023.1277729. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37927445 Free PMC article.
-
Identification of Synaptic DGKθ Interactors That Stimulate DGKθ Activity.Front Synaptic Neurosci. 2022 Apr 27;14:855673. doi: 10.3389/fnsyn.2022.855673. eCollection 2022. Front Synaptic Neurosci. 2022. PMID: 35573662 Free PMC article.
-
Clathrin-independent endocytic retrieval of SV proteins mediated by the clathrin adaptor AP-2 at mammalian central synapses.Elife. 2022 Jan 11;11:e71198. doi: 10.7554/eLife.71198. Elife. 2022. PMID: 35014951 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

