Brentuximab vedotin-containing escalated BEACOPP variants for newly diagnosed advanced-stage classical Hodgkin lymphoma: follow-up analysis of a randomized phase II study from the German Hodgkin Study Group
- PMID: 34408266
- PMCID: PMC8807388
- DOI: 10.1038/s41375-021-01386-z
Brentuximab vedotin-containing escalated BEACOPP variants for newly diagnosed advanced-stage classical Hodgkin lymphoma: follow-up analysis of a randomized phase II study from the German Hodgkin Study Group
Conflict of interest statement
BvT is an advisor or consultant for Amgen, BMS/Celgene, Novartis, Pentixafarm, Pfizer, Takeda, Merck Sharp & Dohme, and Gilead Kite; has received honoraria from Novartis, Roche Pharma AG, Takeda, and Merck Sharp & Dohme; reports research funding from Novartis (Inst), Merck Sharp & Dohme (Inst), and Takeda (Inst); and reports travel support from AbbVie, AstraZeneca, KiteGilead, Merck Sharp & Dohme, Takeda, and Novartis. AZ has received honoraria from Takeda; and reports travel grants from Takeda. All other authors report no potential conflicts of interest.
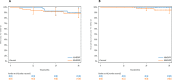
Figures
References
-
- Engert A, Haverkamp H, Kobe C, Markova J, Renner C, Ho A, et al. Reduced-intensity chemotherapy and PET-guided radiotherapy in patients with advanced stage Hodgkin’s lymphoma (HD15 trial): a randomised, open-label, phase 3 non-inferiority trial. Lancet. 2012;379:1791–9. doi: 10.1016/S0140-6736(11)61940-5. - DOI - PubMed
-
- Borchmann P, Goergen H, Kobe C, Lohri A, Greil R, Eichenauer DA, et al. PET-guided treatment in patients with advanced-stage Hodgkin’s lymphoma (HD18): final results of an open-label, international, randomised phase 3 trial by the German Hodgkin Study Group. Lancet. 2017. 10.1016/S0140-6736(17)32134-7. - PubMed
-
- Eichenauer DA, Becker I, Monsef I, Chadwick N, de Sanctis V, Federico M, et al. Secondary malignant neoplasms, progression-free survival and overall survival in patients treated for Hodgkin lymphoma: a systematic review and meta-analysis of randomized clinical trials. Haematologica. 2017;102:1748–57. doi: 10.3324/haematol.2017.167478. - DOI - PMC - PubMed
-
- Eichenauer DA, Plutschow A, Kreissl S, Sokler M, Hellmuth JC, Meissner J, et al. Incorporation of brentuximab vedotin into first-line treatment of advanced classical Hodgkin’s lymphoma: final analysis of a phase 2 randomised trial by the German Hodgkin Study Group. Lancet Oncol. 2017;18:1680–7. doi: 10.1016/S1470-2045(17)30696-4. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical