Dietary fructose improves intestinal cell survival and nutrient absorption
- PMID: 34408323
- PMCID: PMC8686685
- DOI: 10.1038/s41586-021-03827-2
Dietary fructose improves intestinal cell survival and nutrient absorption
Abstract
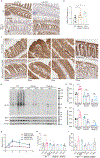
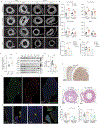
Fructose consumption is linked to the rising incidence of obesity and cancer, which are two of the leading causes of morbidity and mortality globally1,2. Dietary fructose metabolism begins at the epithelium of the small intestine, where fructose is transported by glucose transporter type 5 (GLUT5; encoded by SLC2A5) and phosphorylated by ketohexokinase to form fructose 1-phosphate, which accumulates to high levels in the cell3,4. Although this pathway has been implicated in obesity and tumour promotion, the exact mechanism that drives these pathologies in the intestine remains unclear. Here we show that dietary fructose improves the survival of intestinal cells and increases intestinal villus length in several mouse models. The increase in villus length expands the surface area of the gut and increases nutrient absorption and adiposity in mice that are fed a high-fat diet. In hypoxic intestinal cells, fructose 1-phosphate inhibits the M2 isoform of pyruvate kinase to promote cell survival5-7. Genetic ablation of ketohexokinase or stimulation of pyruvate kinase prevents villus elongation and abolishes the nutrient absorption and tumour growth that are induced by feeding mice with high-fructose corn syrup. The ability of fructose to promote cell survival through an allosteric metabolite thus provides additional insights into the excess adiposity generated by a Western diet, and a compelling explanation for the promotion of tumour growth by high-fructose corn syrup.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Figures

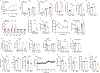









Comment in
-
Fructose in the diet expands the surface of the gut and promotes nutrient absorption.Nature. 2021 Sep;597(7875):180-182. doi: 10.1038/d41586-021-02195-1. Nature. 2021. PMID: 34408303 No abstract available.
-
Dietary fructose acts on gut to increase nutrient uptake.Nat Rev Endocrinol. 2021 Nov;17(11):639. doi: 10.1038/s41574-021-00561-7. Nat Rev Endocrinol. 2021. PMID: 34453128 No abstract available.
-
Untangling the Contribution of Fructose Metabolism to Obesity and Colorectal Cancer: A Tale of Adaptation to Hypoxia in the Gut.Gastroenterology. 2022 Oct;163(4):1117-1118. doi: 10.1053/j.gastro.2022.06.022. Epub 2022 Jun 10. Gastroenterology. 2022. PMID: 35697138 No abstract available.
References
-
- Bray GA, Nielsen SJ & Popkin BM Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am. J. Clin. Nutr 79, 537–543 (2004). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

