Are Dilated Fundus Examinations Needed for OCT-Guided Retreatment of Exudative Age-Related Macular Degeneration? A Prospective, Randomized, Pilot Study
- PMID: 34408396
- PMCID: PMC8367217
- DOI: 10.2147/OPTH.S315554
Are Dilated Fundus Examinations Needed for OCT-Guided Retreatment of Exudative Age-Related Macular Degeneration? A Prospective, Randomized, Pilot Study
Abstract
Purpose: To report findings when dilated fundus examination (DFE) is omitted from follow-up of patients receiving anti-VEGF injections for neovascular age-related macular degeneration (NVAMD).
Design: Randomized pilot study.
Participants: NVAMD patients with two or more injections of anti-VEGF within prior six months who were expected to require treatment for at least eight more months.
Methods and interventions: Participants were assigned to either retinal imaging and DFE or retinal imaging without a DFE except at 16 weeks and 32 weeks and at study completion.
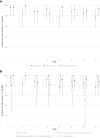
Outcomes: Primary safety outcomes were change in usual-corrected visual acuity (UCVA) and central subfield thickness (CST). Primary efficacy outcomes included time spent in clinic and patient satisfaction with clinic visits.
Results: The 66 participants had mean baseline UCVA of 20/50 in the study eye. Median change in UCVA from baseline to each clinic visit in each arm was "no change". Mean change in CST was less than 15 microns from baseline to any follow-up clinic visit. Time spent in the clinic at follow-up visits averaged 20 minutes less for participants in the Imaging Only group than those in the Full Exam group. More participants in the Imaging Only group were satisfied with the time spent in clinic and with the clinic visits overall than participants in the Full Exam group: means of 71 vs 91 minutes, respectively, per clinic visit.
Conclusion: Based on findings from this randomized pilot study, follow-up retina clinic visits for established patients who have NVAMD and are under treatment with intravitreous injection of anti-VEGF agents could be streamlined by implementing longer intervals between DFE and by relying on imaging alone to make most decisions regarding the need for retreatment, thereby reducing the time spent by patients in clinic and increasing their satisfaction with care received, without excess adverse events.
Trial registration: NCT02251366.
Keywords: anti-VEGF injection; neovascular age-related macular degeneration; time in clinic.
© 2021 Solomon et al.
Conflict of interest statement
Ms. Kyerematen and Ms. Qutab were supported by a grant from the Wilmer Pooled Professors Research Fund, 2014–2016. Dr. Hawkins and Dr. Solomon received support from an unrestricted institutional grant to the Wilmer Eye Institute from Research to Prevent Blindness, New York, NY. Dr. Solomon also received support from the Katharine M. Graham Professorship in Ophthalmology. Ms. Wang and Mr. Dreger received support from a core grant, P30EY001765, to the Wilmer Eye Institute from the National Eye Institute, National Institutes of Health, US Department of Health and Human Services, Bethesda, Maryland. Dr Adam S Wenick reports personal fees from Genentech, outside the submitted work. Ms Jiangxia Wang reports grants from NEI, during the conduct of the study. The authors report no other conflicts of interest in this work.
Figures



References
-
- Maguire MG, Martin DF, Ying G-S; Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group. Five-Year outcomes with anti-vascular endothelial growth factor treatment of neovascular age-related macular degeneration: the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2016;123(8):1751–1761. doi:10.1016/j.ophtha.2016.03.045 - DOI - PMC - PubMed
Publication types
Associated data
LinkOut - more resources
Full Text Sources
Medical

