Myeloprotective Effects of Trilaciclib Among Patients with Small Cell Lung Cancer at Increased Risk of Chemotherapy-Induced Myelosuppression: Pooled Results from Three Phase 2, Randomized, Double-Blind, Placebo-Controlled Studies
- PMID: 34408488
- PMCID: PMC8363477
- DOI: 10.2147/CMAR.S313045
Myeloprotective Effects of Trilaciclib Among Patients with Small Cell Lung Cancer at Increased Risk of Chemotherapy-Induced Myelosuppression: Pooled Results from Three Phase 2, Randomized, Double-Blind, Placebo-Controlled Studies
Abstract
Purpose: Trilaciclib is an intravenous cyclin-dependent kinase 4/6 inhibitor indicated to decrease the incidence of chemotherapy-induced myelosuppression (CIM) by protecting hematopoietic stem and progenitor cells and immune system function from chemotherapy-induced damage (myeloprotection). Here, we investigated the myeloprotective effects of trilaciclib among patients at increased risk of CIM.
Patients and methods: Data were pooled from three randomized, double-blind, placebo-controlled, phase 2 clinical studies of trilaciclib administered prior to chemotherapy in patients with extensive-stage small cell lung cancer (ES-SCLC). Myeloprotective outcomes were evaluated in patient subgroups based on age (<65 or ≥65 years), risk of chemotherapy-induced febrile neutropenia (FN), and risk of anemia or red blood cell (RBC) transfusions. For the FN and anemia analyses, risk factors were identified from published literature and used to classify patients into FN and anemia risk categories. Subgroup analysis based on age was also performed on patient reported outcome (PRO) measures.
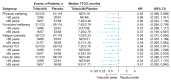
Results: In total, 123 patients received trilaciclib and 119 patients received placebo. Myeloprotective benefits of trilaciclib were observed regardless of age, with greater effects observed among patients aged ≥65 years. Across FN risk factors and categories, trilaciclib had beneficial effects on neutrophil-related endpoints vs placebo, with greater effects observed in patients at higher risk of FN. Effects on RBC-related endpoints favored trilaciclib vs placebo, regardless of anemia risk factors and categories. Improvements in PROs with trilaciclib were observed irrespective of age group, but with greater improvements and less deterioration from baseline observed in older patients.
Conclusion: By both decreasing the incidence of CIM and improving quality of life, trilaciclib has the potential to allow patients receiving chemotherapy for ES-SCLC, including patients who are older or more vulnerable to CIM, to receive chemotherapy on schedule and at standard-of-care doses, and to improve the experience for patients receiving chemotherapy to treat ES-SCLC.
Clinical trial numbers: NCT02499770; NCT03041311; NCT02514447.
Keywords: chemotherapy; myelopreservation; myeloprotection; myelosuppression; small cell lung cancer; trilaciclib.
© 2021 Hussein et al.
Conflict of interest statement
Dr Maen Hussein reports personal fees from Integra Connect, Coherus Biosciences, Athenex, Karyopharm Therapeutics, Bristol-Myers Squibb, and AstraZeneca, outside the submitted work. Dr Alexander Spira has received research funding from G1 Therapeutics, Inc., has been a consultant to Amgen, AstraZeneca, Bristol-Myers Squibb, Merck, Mirati, and Novartis, and received personal fees from Sanofi, AstraZeneca and Bristol-Myers Squibb. Dr Yili Pritchett and Dr Rajesh Malik are paid employees and shareowners of G1 Therapeutics, Inc. Dr Joyce M. Antal was an employee of G1 Therapeutics, Inc. at the time of manuscript preparation. Dr J. Thaddeus Beck has received grants from Abbvie, Alliance, Amgen, Ascentage Pharma Group, AstraZeneca, Bayer, Biodesix, Boehringer Ingelheim, Boston Biomedical, Bristol-Myers Squibb, Calithera, Celgene, Daiichi Sankyo, EMD Serono, Eli Lilly, Evelo, Exact Sciences, G1 Therapeutics, Inc., Genentech-Roche, Hutchison, Immunomedics, Janssen, Laekna, Mirati Therapeutics, MT Group, Nektar, Novartis, Novocure, Oncopeptides, Pfizer, Polynoma, Seattle Genetics, Syneos Health, Tarveda Therapeutics, Tesaro, TG Therapeutics, Ultimovacs, and Vaccinex. The authors report no other conflicts of interest in this work.
Figures


References
-
- Bryer E, Henry D. Chemotherapy-induced anemia: etiology, pathophysiology, and implications for contemporary practice. Int J Clin Transfus Med. 2018;6:21–31. doi:10.2147/IJCTM.S187569 - DOI
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

