Chronic Ethanol Exposure Enhances Facial Stimulation-Evoked Mossy Fiber-Granule Cell Synaptic Transmission via GluN2A Receptors in the Mouse Cerebellar Cortex
- PMID: 34408633
- PMCID: PMC8365521
- DOI: 10.3389/fnsys.2021.657884
Chronic Ethanol Exposure Enhances Facial Stimulation-Evoked Mossy Fiber-Granule Cell Synaptic Transmission via GluN2A Receptors in the Mouse Cerebellar Cortex
Abstract
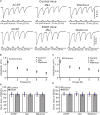
Sensory information is transferred to the cerebellar cortex via the mossy fiber-granule cell (MF-GC) pathway, which participates in motor coordination and motor learning. We previously reported that chronic ethanol exposure from adolescence facilitated the sensory-evoked molecular layer interneuron-Purkinje cell synaptic transmission in adult mice in vivo. Herein, we investigated the effect of chronic ethanol exposure from adolescence on facial stimulation-evoked MF-GC synaptic transmission in the adult mouse cerebellar cortex using electrophysiological recording techniques and pharmacological methods. Chronic ethanol exposure from adolescence induced an enhancement of facial stimulation-evoked MF-GC synaptic transmission in the cerebellar cortex of adult mice. The application of an N-methyl-D-aspartate receptor (NMDAR) antagonist, D-APV (250 μM), induced stronger depression of facial stimulation-evoked MF-GC synaptic transmission in chronic ethanol-exposed mice compared with that in control mice. Chronic ethanol exposure-induced facilitation of facial stimulation evoked by MF-GC synaptic transmission was abolished by a selective GluN2A antagonist, PEAQX (10 μM), but was unaffected by the application of a selective GluN2B antagonist, TCN-237 (10 μM), or a type 1 metabotropic glutamate receptor blocker, JNJ16259685 (10 μM). These results indicate that chronic ethanol exposure from adolescence enhances facial stimulation-evoked MF-GC synaptic transmission via GluN2A, which suggests that chronic ethanol exposure from adolescence impairs the high-fidelity transmission capability of sensory information in the cerebellar cortex by enhancing the NMDAR-mediated components of MF-GC synaptic transmission in adult mice in vivo.
Keywords: N-methyl-D-aspartate receptors; cerebellar cortex; chronic ethanol exposure; in vivo electrophysiological recording; mossy fiber-granule cell synaptic transmission; sensory stimulation.
Copyright © 2021 Li, Dong, Li, Zhang, Bing, Chu, Cui and Qiu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Bhattacharya S., Khatri A., Swanger S. A., DiRaddo J. O., Yi F., Hansen K. B., et al. (2018). Triheteromeric GluN1/GluN2A/GluN2C NMDARs with unique single-channel properties are the dominant receptor population in cerebellar granule cells. Neuron 99 315–328.e5. 10.1016/j.neuron.2018.06.010 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

