Age-Associated Neurological Complications of COVID-19: A Systematic Review and Meta-Analysis
- PMID: 34408638
- PMCID: PMC8366271
- DOI: 10.3389/fnagi.2021.653694
Age-Associated Neurological Complications of COVID-19: A Systematic Review and Meta-Analysis
Abstract
The outbreak of the novel and highly infectious severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has resulted in hundreds of millions of infections and millions of deaths globally. Infected individuals that progress to coronavirus disease-19 (COVID-19) experience upper and lower respiratory complications that range in severity and may lead to wide-spread inflammation and generalized hypoxia or hypoxemia that impacts multiple organ systems, including the central and peripheral nervous systems. Since the SARS-CoV-2 outbreak, multiple reports continue to emerge that detail neurological symptoms, ranging from relatively mild (e.g., impaired taste and/or smell) to severe (e.g., stroke), suggesting SARS-CoV-2 may be neurotropic and/or contribute to nervous system injury through direct and/or indirect mechanisms. To gain insight into the types of neurological complications associated with SARS-CoV-2 infection and their possible relationship with age, sex, COVID-19 severity, and comorbidities, we performed a systematic review of case reports and series published in 2020 - April 4, 2021 of infected patients with neurological manifestations. Meta-analyses were conducted using individual patient data from reports where these data could be extracted. Here, we report neurological injury occurs across the lifespan in the context of infection, with and without known comorbidities, and with all disease severities, including asymptomatic patients. Older individuals, however, are more susceptible to developing life-threatening COVID-19 and cerebrovascular disease (CVD), such as stroke. A mild but inverse correlation with age was seen with CNS inflammatory diseases, such as encephalitis, as well as taste and/or smell disorders. When reported, increased age was also associated with comorbid cardiovascular risk factors, including hypertension, diabetes mellitus, and lipid disorders, but not with obesity. Obesity did correlate with development of critical COVID-19. Discussion into potential pathophysiological mechanisms by which neurological symptoms arise and long-term consequences of infection to the nervous system is also provided.
Keywords: COVID-19; SARS-CoV-2; aging brain; brain; cerebrovascular events; encephalopathy.
Copyright © 2021 Sullivan and Fischer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
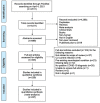
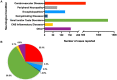
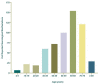
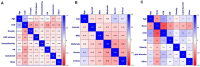
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

