IFN-γ-induced ER stress impairs autophagy and triggers apoptosis in lung cancer cells
- PMID: 34408924
- PMCID: PMC8366549
- DOI: 10.1080/2162402X.2021.1962591
IFN-γ-induced ER stress impairs autophagy and triggers apoptosis in lung cancer cells
Abstract
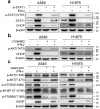
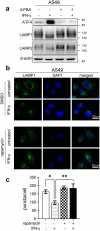
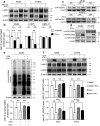
Interferon-gamma (IFN-γ) is a major effector molecule of immunity and a common feature of tumors responding to immunotherapy. Active IFN-γ signaling can directly trigger apoptosis and cell cycle arrest in human cancer cells. However, the mechanisms underlying these actions remain unclear. Here, we report that IFN-γ rapidly increases protein synthesis and causes the unfolded protein response (UPR), as evidenced by the increased expression of glucose-regulated protein 78, activating transcription factor-4, and c/EBP homologous protein (CHOP) in cells treated with IFN-γ. The JAK1/2-STAT1 and AKT-mTOR signaling pathways are required for IFN-γ-induced UPR. Endoplasmic reticulum (ER) stress promotes autophagy and restores homeostasis. Surprisingly, in IFN-γ-treated cells, autophagy was impaired at the step of autophagosome-lysosomal fusion and caused by a significant decline in the expression of lysosomal membrane protein-1 and -2 (LAMP-1/LAMP-2). The ER stress inhibitor 4-PBA restored LAMP expression in IFN-γ-treated cells. IFN-γ stimulation activated the protein kinase-like ER kinase (PERK)-eukaryotic initiation factor 2a subunit (eIF2α) axis and caused a reduction in global protein synthesis. The PERK inhibitor, GSK2606414, partially restored global protein synthesis and LAMP expression in cells treated with IFN-γ. We further investigated the functional consequences of IFN-γ-induced ER stress. We show that inhibition of ER stress significantly prevents IFN-γ-triggered apoptosis. CHOP knockdown abrogated IFN-γ-mediated apoptosis. Inhibition of ER stress also restored cyclin D1 expression in IFN-γ-treated cells. Thus, ER stress and the UPR caused by IFN-γ represent novel mechanisms underlying IFN-γ-mediated anticancer effects. This study expands our understanding of IFN-γ-mediated signaling and its cellular actions in tumor cells.
Keywords: ER stress; IFN-γ; LAMP; apoptosis; autophagy; lung adenocarcinoma.
© 2021 The Author(s). Published with license by Taylor & Francis Group, LLC.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
