Application of Ovarian Cancer Organoids in Precision Medicine: Key Challenges and Current Opportunities
- PMID: 34409036
- PMCID: PMC8366314
- DOI: 10.3389/fcell.2021.701429
Application of Ovarian Cancer Organoids in Precision Medicine: Key Challenges and Current Opportunities
Abstract
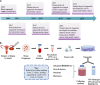
Ovarian cancer (OC) is the leading cause of death among gynecologic malignances. Over the past decades, human-derived models have advanced from monolayer cell cultures to three-dimensional (3D) organoids that could faithfully recapitulate biological characteristics and tumor heterogeneity of primary tissues. As a complement of previous studies based on cell lines or xenografts, organoids provide a 3D platform for mutation-carcinogenesis modeling, high-throughput drug screening, genetic engineering, and biobanking, which might fulfill the gap between basic research and clinical practice. Stepwise, cutting-edge bioengineering techniques of organoid-on-a-chip and 3D bioprinting might converge current challenges and contribute to personalized therapy. We comprehensively reviewed the advantages, challenges, and translational potential of OC organoids. Undeniably, organoids represent an excellent near-physiological platform for OC, paving the way for precision medicine implementation. Future efforts will doubtlessly bring this innovative technique from bench to bedside.
Keywords: organoid; ovarian cancer; precision medicine; preclinical tumor models; three-dimensional platform.
Copyright © 2021 Yang, Huang, Cheng, Jin, Zhang and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

