Biologicals in childhood severe asthma: the European PERMEABLE survey on the status quo
- PMID: 34409097
- PMCID: PMC8365152
- DOI: 10.1183/23120541.00143-2021
Biologicals in childhood severe asthma: the European PERMEABLE survey on the status quo
Abstract
Introduction: Severe asthma is a rare disease in children, for which three biologicals, anti-immunoglobulin E, anti-interleukin-5 and anti-IL4RA antibodies, are available in European countries. While global guidelines exist on who should receive biologicals, knowledge is lacking on how those guidelines are implemented in real life and which unmet needs exist in the field. In this survey, we aimed to investigate the status quo and identify open questions in biological therapy of childhood asthma across Europe.
Methods: Structured interviews regarding experience with biologicals, regulations on access to the different treatment options, drug selection, therapy success and discontinuation of therapy were performed. Content analysis was used to analyse data.
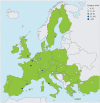
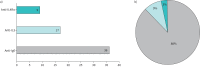
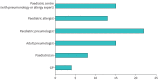
Results: We interviewed 37 experts from 25 European countries and Turkey and found a considerable range in the number of children treated with biologicals per centre. All participating countries provide public access to at least one biological. Most countries allow different medical disciplines to prescribe biologicals to children with asthma, and only a few restrict therapy to specialised centres. We observed significant variation in the time point at which treatment success is assessed, in therapy duration and in the success rate of discontinuation. Most participating centres intend to apply a personalised medicine approach in the future to match patients a priori to available biologicals.
Conclusion: Substantial differences exist in the management of childhood severe asthma across Europe, and the need for further studies on biomarkers supporting selection of biologicals, on criteria to assess therapy response and on how/when to end therapy in stable patients is evident.
Copyright ©The authors 2021.
Conflict of interest statement
Conflict of interest: E. Santos-Valente has nothing to disclose. Conflict of interest: H. Buntrock-Döpke has nothing to disclose. Conflict of interest: R. Abou Taam has nothing to disclose. Conflict of interest: S. Arasi has nothing to disclose. Conflict of interest: A. Bakirtas has nothing to disclose. Conflict of interest: J. Lozano Blasco reports personal fees from Novartis and GSK outside the submitted work. Conflict of interest: K. Bønnelykke has nothing to disclose. Conflict of interest: M. Craiu reports a speaker fee for a scientific meeting regarding 5 years of Xolair in Romania (9 May 2018) from Novartis Romania outside the submitted work. Conflict of interest: R. Cutrera has nothing to disclose. Conflict of interest: A. Deschildre reports personal fees and other support from Novartis, GSK and Sanofi outside the submitted work. Conflict of interest: B. Elnazir has nothing to disclose. Conflict of interest: L. Fleming reports grants from Asthma UK, and speakers fees or fees for expert consultation from Teva, Astra Zeneca, Sanofi, Respiri, Novartis; all fees paid direct to her institution and outside the submitted work. Conflict of interest: U. Frey has nothing to disclose. Conflict of interest: M. Gappa reports personal fees from Boehringer Ingelheim, GSK, Novartis and Sanofi outside the submitted work. Conflict of interest: A. Nieto García reports grants for clinical studies and advisory boards membership, as well as lecture fees from Novartis, GSK and MSD. Conflict of interest: K. Skamstrup Hansen has nothing to disclose. Conflict of interest: L. Hanssens has nothing to disclose. Conflict of interest: K. Jahnz-Rozyk received lecture and advisory board fees from AstraZeneca, Novartis, GSK, and Sanofi Aventis, outside the submitted work. Conflict of interest: M. Jesenak has nothing to disclose. Conflict of interest: S. Kerzel reports a speaker fee from Novartis for a lecture that has no topical overlap at all with the current manuscript. Conflict of interest: M. V. Kopp reports personal fees from ALK-Abello GmbH, Chiesi GmbH, Infectopharm GmbH, Novartis GmbH, Sanofi Aventis HmbH and Vertex GmbH, and grants and personal fees from Allergopharma GmbH, outside the submitted work. Conflict of interest: G.H. Koppelman reports grants from Lung Foundation Netherlands, TETRI Foudation, Ubbo Emmius Foundation, Vertex, Teva the Netherlands, GSK, European Union, outside the submitted work; and he participated in advisory board meetings to GSK and Pure IMS (money to institution). Conflict of interest: U. Krivec has nothing to disclose. Conflict of interest: K.A. MacLeod has nothing to disclose. Conflict of interest: M. Mäkelä has nothing to disclose. Conflict of interest: E. Melén reports personal fees from AstraZeneca, Chiesi, Novartis and Sanofi (advisory board fees), outside the submitted work. Conflict of interest: G. Mezei reports a travel grant from LOFARMA outside the submitted work. Conflict of interest: A. Moeller has nothing to disclose. Conflict of interest: A. Moreira has nothing to disclose. Conflict of interest: P. Pohunek reports personal fees for advisory board membership from Novartis and GlaxoSmithKline, and for consultation from Chiesi and AstraZeneca, outside the submitted work. Conflict of interest: P. Minić has nothing to disclose. Conflict of interest: N.W.P. Rutjes reports personal fees from GSK (advisory board on mepolizumab) and Sanofi (advisory board on dupilumab) outside the submitted work. Conflict of interest: P. Sammut has nothing to disclose. Conflict of interest: N. Schwerk reports lecture and advisory board fees from Novartis, Sanofi and Allergopharma; advisory board fees from Boehringer Ingelheim; and lecture fees from Abvie and Infectopharm, all outside the submitted work. Conflict of interest: Z. Szépfalusi has nothing to disclose. Conflict of interest: M. Turkalj has nothing to disclose. Conflict of interest: I. Tzotcheva has nothing to disclose. Conflict of interest: A. Ulmeanu has nothing to disclose. Conflict of interest: S. Verhulst reports grants from GSK during the conduct of the study. Conflict of interest: P. Xepapadaki reports personal fees for advisory services from Uriach, Novartis, Nestle and Nutricia outside the submitted work. Conflict of interest: J. Niggel has nothing to disclose. Conflict of interest: S. Vijverberg reports grants from ZonMW during the conduct of the study. Conflict of interest: A-H. Maitland van der Zee has received research grants outside the submitted work from GSK, Boehringer Ingelheim and Vertex; she is the principal investigator of a P4O2 (Precision Medicine for more Oxygen) public–private partnership sponsored by Health Holland involving many private partners that contribute in cash and/or in kind (Boehringer Ingelheim, Breathomix, Fluidda, Ortec Logiqcare, Philips, Quantib-U, Smartfish, SODAQ, Thirona, TopMD and Novartis); and she has served in advisory boards for AstraZeneca, GSK and Boehringer Ingelheim, with money paid to her institution. Conflict of interest: U. Potocnik was funded by the Ministry of Education, Science and Sport of the Republic of Slovenia, grant PERMEABLE (contract number C3330-19-252012), during the conduct of the study. Conflict of interest: S. M. Reinartz has nothing to disclose. Conflict of interest: C. M. van Drunen has nothing to disclose. Conflict of interest: M. Kabesch reports grants to his institution from the European Union, the German Ministry of Education and Research, and the German Research Foundation, during the conduct of the study; and consultancy fees from Bionorica, Sanofi, Novartis and Bencard, payments for lectures form the ERS, EAACI, ATS, Novartis, Glaxo, Nutricia, Hipp and Allergopharma, and a patent for a method for testing a subject thought to have or be disposed to asthma (European patent application 5 EP070301 135.5), outside the submitted work.
Figures

Comment in
-
Mepolizumab Treatment in Severe Pediatric Asthma: First Multicentric Real-World Data.Klin Padiatr. 2022 Sep;234(5):305-308. doi: 10.1055/a-1717-2234. Epub 2022 Mar 22. Klin Padiatr. 2022. PMID: 35319087 English. No abstract available.
References
-
- Global Initiative for Asthma . Global Strategy for Asthma Management and Prevention, 2020. www.ginasthma.org Date last accessed: 20 July 2020. Date last updated: 26 April 2021.
LinkOut - more resources
Full Text Sources
