Psychosis Biotypes: Replication and Validation from the B-SNIP Consortium
- PMID: 34409449
- PMCID: PMC8781330
- DOI: 10.1093/schbul/sbab090
Psychosis Biotypes: Replication and Validation from the B-SNIP Consortium
Abstract
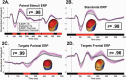
Current clinical phenomenological diagnosis in psychiatry neither captures biologically homologous disease entities nor allows for individualized treatment prescriptions based on neurobiology. In this report, we studied two large samples of cases with schizophrenia, schizoaffective, and bipolar I disorder with psychosis, presentations with clinical features of hallucinations, delusions, thought disorder, affective, or negative symptoms. A biomarker approach to subtyping psychosis cases (called psychosis Biotypes) captured neurobiological homology that was missed by conventional clinical diagnoses. Two samples (called "B-SNIP1" with 711 psychosis and 274 healthy persons, and the "replication sample" with 717 psychosis and 198 healthy persons) showed that 44 individual biomarkers, drawn from general cognition (BACS), motor inhibitory (stop signal), saccadic system (pro- and anti-saccades), and auditory EEG/ERP (paired-stimuli and oddball) tasks of psychosis-relevant brain functions were replicable (r's from .96-.99) and temporally stable (r's from .76-.95). Using numerical taxonomy (k-means clustering) with nine groups of integrated biomarker characteristics (called bio-factors) yielded three Biotypes that were virtually identical between the two samples and showed highly similar case assignments to subgroups based on cross-validations (88.5%-89%). Biotypes-1 and -2 shared poor cognition. Biotype-1 was further characterized by low neural response magnitudes, while Biotype-2 was further characterized by overactive neural responses and poor sensory motor inhibition. Biotype-3 was nearly normal on all bio-factors. Construct validation of Biotype EEG/ERP neurophysiology using measures of intrinsic neural activity and auditory steady state stimulation highlighted the robustness of these outcomes. Psychosis Biotypes may yield meaningful neurobiological targets for treatments and etiological investigations.
Keywords: Biotypes; EEG; biomarkers; classification; cognition; psychosis; saccades.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Maryland Psychiatric Research Center.
Figures




References
-
- Biedermann F, Fleischhacker WW. Psychotic disorders in DSM-5 and ICD-11. CNS Spectr. 2016;21(4):349–354. - PubMed
-
- American Psychiatric Association., American Psychiatric Association. DSM-5 Task Force. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. American Psychiatric Association; 2013:xliv; 947 p.
-
- Hyman SE. The diagnosis of mental disorders: the problem of reification. Annu Rev Clin Psychol. 2010;6:155–179. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 MH096957/MH/NIMH NIH HHS/United States
- R01 MH103368/MH/NIMH NIH HHS/United States
- R01 MH096942/MH/NIMH NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- TL1 TR002382/TR/NCATS NIH HHS/United States
- R01 MH077851/MH/NIMH NIH HHS/United States
- UL1 TR002378/TR/NCATS NIH HHS/United States
- R01 MH103366/MH/NIMH NIH HHS/United States
- R01 MH078113/MH/NIMH NIH HHS/United States
- R01 MH096900/MH/NIMH NIH HHS/United States
- R01 MH096913/MH/NIMH NIH HHS/United States
- R01 MH077945/MH/NIMH NIH HHS/United States

