Evaluation of the Khorana, PROTECHT, and 5-SNP scores for prediction of venous thromboembolism in patients with cancer
- PMID: 34409743
- PMCID: PMC9291564
- DOI: 10.1111/jth.15503
Evaluation of the Khorana, PROTECHT, and 5-SNP scores for prediction of venous thromboembolism in patients with cancer
Abstract
Background: The Khorana score is a validated tool to identify cancer patients at higher risk of venous thromboembolism (VTE).
Objective: We compared its predictive performance to that of the clinical PROTECHT and the polygenic 5-SNP scores in patients who participated in the Dutch CPCT-02 study.
Patients/methods: Data on VTE and its risk factors were retrospectively collected for 2729 patients with advanced stage solid tumors planned for systemic cancer treatment. Patients were followed for 6 months. Overall discriminatory performance of the scores was evaluated by time-dependent c-indices. The scores were additionally evaluated dichotomously in competing risk models.
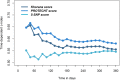
Results: A total of 160 (5.9%) patients developed VTE during follow-up. Time-dependent c-indices at 6 months for the Khorana, PROTECHT, and 5-SNP scores were 0.57 (95% confidence interval [CI]: 0.55-0.60), 0.60 (95% CI: 0.57-0.62), and 0.54 (95% CI: 0.51-0.57), respectively. The dichotomous scores classified 9.6%, 16.8%, and 9.5% as high-risk, respectively. VTE risk was about 2-fold higher among high-risk patients than low-risk patients for the Khorana (subdistribution hazard ratio [SHR] 1.9, 95% CI: 1.3-3.0), PROTECHT (SHR 2.1, 95% CI: 1.5-3.0), and 5-SNP scores (SHR 1.7, 95% CI: 1.03-2.8). The sensitivity at 6 months was 16.6% (95% CI: 10.5-22.7), 28.9% (95% CI: 21.5-36.3), and 14.9% (95% CI: 8.5-21.2), respectively.
Conclusions: Performance of the PROTECHT or 5-SNP score was not superior to that of the Khorana score. The majority of cancer patients who developed VTE during 6-month follow-up were not identified by these scores. Future directions for studies on cancer-associated VTE prediction may include combined clinical-genetic scores.
Keywords: neoplasms; polymorphism; risk assessment; single nucleotide; thrombosis; venous thromboembolism.
© 2021 The Authors. Journal of Thrombosis and Haemostasis published by Wiley Periodicals LLC on behalf of International Society on Thrombosis and Haemostasis.
Conflict of interest statement
N.A.M. Guman, R.J. van Geffen, F.I. Mulder, T.F. van Haaps, V. Hovsepjan, G.A. Cirkel, A.J. ten Tije, L.V. Beerepoot, V.C.G. Tjan‐Heijnen, A.J.E. Vulink, M. Los, A.H. Zwinderman, B. Ferwerda, and N. Steeghs have no conflicts of interest to declare. M. Labots reports consulting fees from BMS, which were transferred to her institution. F.Y.F.L. de Vos reports a research grant from the Foundation STOPbraintumors.org and financial research support from AbbVie, BMS, Novartis, EORTC, Vaximm, BioClin Therapeutics. He participated on a Data Safety Monitoring Board in a clinical trial on chloroquinine in glioblastoma treatment at the Maastricht University Medical Center and is a faculty member of the ESMO CNS tumors, the Quality of Care commission Dutch Society of Medical Oncology, and the Quality Assurance commission EORTC. H.M.W. van Laarhoven has received research funding from BMS, Lilly, MSD, Nordic Pharma, Servier, and Bayer. She participated on Data Safety Monitoring Boards for BMS, Lilly, MSD, Nordic Pharma, and Servier, and received consulting fees from these parties. P. Hamberg reports consulting fees from Astellas, MSD, Pfizer AstraZeneca, BMS, Ipsen. M.P.J.K. Lolkema reports grants from JnJ, Astellas, MSD, and Sanofi; consulting fees from Roche, Bayer, Amgen, JnJ, Sanofi, Servier, Pfizer, Incyte, and Novartis; and support for attending meetings and/or travel from MSD, Sanofi, and Servier. H.R. Büller reports personal fees from Daiichi Sankyo, Bayer Healthcare, BMS/Pfizer, Boehringer Ingelheim, Portola, Medscape, Eli Lilly, Sanofi Aventis, and Ionis. P.W. Kamphuisen has received research grants from Daiichi Sankyo and Roche Diagnostics and the Tergooi Academy. N. van Es reports advisory board honoraria from Daiichi‐Sankyo, Bayer, and LEO Pharma, which were transferred to his institute.
Figures

References
-
- Sørensen HT, Mellemkjær L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343:1846‐1850. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

