A Retrospective Test-Negative Case-Control Study to Evaluate Influenza Vaccine Effectiveness in Preventing Hospitalizations in Children
- PMID: 34410341
- PMCID: PMC8599178
- DOI: 10.1093/cid/ciab709
A Retrospective Test-Negative Case-Control Study to Evaluate Influenza Vaccine Effectiveness in Preventing Hospitalizations in Children
Abstract
Background: Vaccination is the primary strategy to reduce influenza burden. Influenza vaccine effectiveness (VE) can vary annually depending on circulating strains.
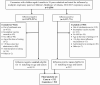
Methods: We used a test-negative case-control study design to estimate influenza VE against laboratory-confirmed influenza-related hospitalizations among children (aged 6 months-17 years) across 5 influenza seasons in Atlanta, Georgia, from 2012-2013 to 2016-2017. Influenza-positive cases were randomly matched to test-negative controls based on age and influenza season in a 1:1 ratio. We used logistic regression models to compare odds ratios (ORs) of vaccination in cases to controls. We calculated VE as [100% × (1 - adjusted OR)] and computed 95% confidence intervals (CIs) around the estimates.
Results: We identified 14 596 hospitalizations of children who were tested for influenza using the multiplex respiratory molecular panel; influenza infection was detected in 1017 (7.0%). After exclusions, we included 512 influenza-positive cases and 512 influenza-negative controls. The median age was 5.9 years (interquartile range, 2.7-10.3), 497 (48.5%) were female, 567 (55.4%) were non-Hispanic Black, and 654 (63.9%) children were unvaccinated. Influenza A accounted for 370 (72.3%) of 512 cases and predominated during all 5 seasons. The adjusted VE against influenza-related hospitalizations during 2012-2013 to 2016-2017 was 51.3% (95% CI, 34.8% to 63.6%) and varied by season. Influenza VE was 54.7% (95% CI, 37.4% to 67.3%) for influenza A and 37.1% (95% CI, 2.3% to 59.5%) for influenza B.
Conclusions: Influenza vaccination decreased the risk of influenza-related pediatric hospitalizations by >50% across 5 influenza seasons.
Keywords: adolescent; immunization; influenza vaccine effectiveness; pediatric.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
References
-
- Thompson WW, Shay DK, Weintraub E, et al. . Influenza-associated hospitalizations in the United States. JAMA 2004; 292:1333-40. - PubMed
-
- Ferdinands JM, Fry AM, Reynolds S, et al. . Intraseason waning of influenza vaccine protection: evidence from the US Influenza Vaccine Effectiveness Network, 2011-12 through 2014-15. Clin Infect Dis 2017; 64:544-50. - PubMed
-
- Petrie JG, Ohmit SE, Truscon R, et al. . Modest waning of influenza vaccine efficacy and antibody titers during the 2007-2008 influenza season. J Infect Dis 2016; 214: 1142-9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

