Current state of and need for enzyme engineering of 2-deoxy-D-ribose 5-phosphate aldolases and its impact
- PMID: 34410440
- PMCID: PMC8403123
- DOI: 10.1007/s00253-021-11462-0
Current state of and need for enzyme engineering of 2-deoxy-D-ribose 5-phosphate aldolases and its impact
Abstract
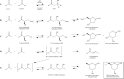
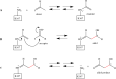
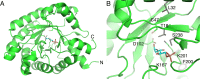
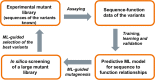
Deoxyribose-5-phosphate aldolases (DERAs, EC 4.1.2.4) are acetaldehyde-dependent, Class I aldolases catalyzing in nature a reversible aldol reaction between an acetaldehyde donor (C2 compound) and glyceraldehyde-3-phosphate acceptor (C3 compound, C3P) to generate deoxyribose-5-phosphate (C5 compound, DR5P). DERA enzymes have been found to accept also other types of aldehydes as their donor, and in particular as acceptor molecules. Consequently, DERA enzymes can be applied in C-C bond formation reactions to produce novel compounds, thus offering a versatile biocatalytic alternative for synthesis. DERA enzymes, found in all kingdoms of life, share a common TIM barrel fold despite the low overall sequence identity. The catalytic mechanism is well-studied and involves formation of a covalent enzyme-substrate intermediate. A number of protein engineering studies to optimize substrate specificity, enzyme efficiency, and stability of DERA aldolases have been published. These have employed various engineering strategies including structure-based design, directed evolution, and recently also machine learning-guided protein engineering. For application purposes, enzyme immobilization and usage of whole cell catalysis are preferred methods as they improve the overall performance of the biocatalytic processes, including often also the stability of the enzyme. Besides single-step enzymatic reactions, DERA aldolases have also been applied in multi-enzyme cascade reactions both in vitro and in vivo. The DERA-based applications range from synthesis of commodity chemicals and flavours to more complicated and high-value pharmaceutical compounds. KEY POINTS: • DERA aldolases are versatile biocatalysts able to make new C-C bonds. • Synthetic utility of DERAs has been improved by protein engineering approaches. • Computational methods are expected to speed up the future DERA engineering efforts.
Keywords: Aldolase; Applications; Biocatalysis; C–C bond formation; DERA; Protein engineering.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Alford RF, Leaver-Fay A, Jeliazkov JR, O’Meara MJ, DiMaio FP, Park H, Shapovalov MV, Renfrew PD, Mulligan VK, Kappel K, Labonte JW, Pacella MS, Bonneau R, Bradley P, Dunbrack RL, Das R, Baker D, Kuhlman B, Kortemme T, Gray JJ. The Rosetta all-atom energy function for macromolecular modeling and design. J Chem Theory Comput. 2017;13:3031–3048. doi: 10.1021/acs.jctc.7b00125. - DOI - PMC - PubMed
-
- Barbas CF, Wang YF, Wong CH. Deoxyribose-5-phosphate aldolase as a synthetic catalyst. J Am Chem Soc. 1990;112:2013–2014. doi: 10.1021/ja00161a064. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

