Exploring the missing heritability in subjects with hearing loss, enlarged vestibular aqueducts, and a single or no pathogenic SLC26A4 variant
- PMID: 34410491
- PMCID: PMC9035008
- DOI: 10.1007/s00439-021-02336-6
Exploring the missing heritability in subjects with hearing loss, enlarged vestibular aqueducts, and a single or no pathogenic SLC26A4 variant
Erratum in
-
Correction to: Exploring the missing heritability in subjects with hearing loss, enlarged vestibular aqueducts, and a single or no pathogenic SLC26A4 variant.Hum Genet. 2022 Apr;141(3-4):991. doi: 10.1007/s00439-021-02377-x. Hum Genet. 2022. PMID: 34608567 Free PMC article. No abstract available.
Abstract
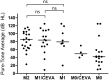
Pathogenic variants in SLC26A4 have been associated with autosomal recessive hearing loss (arHL) and a unilateral or bilateral enlarged vestibular aqueduct (EVA). SLC26A4 is the second most frequently mutated gene in arHL. Despite the strong genotype-phenotype correlation, a significant part of cases remains genetically unresolved. In this study, we investigated a cohort of 28 Dutch index cases diagnosed with HL in combination with an EVA but without (M0) or with a single (M1) pathogenic variant in SLC26A4. To explore the missing heritability, we first determined the presence of the previously described EVA-associated haplotype (Caucasian EVA (CEVA)), characterized by 12 single nucleotide variants located upstream of SLC26A4. We found this haplotype and a delimited V1-CEVA haplotype to be significantly enriched in our M1 patient cohort (10/16 cases). The CEVA haplotype was also present in two M0 cases (2/12). Short- and long-read whole genome sequencing and optical genome mapping could not prioritize any of the variants present within the CEVA haplotype as the likely pathogenic defect. Short-read whole-genome sequencing of the six M1 cases without this haplotype and the two M0/CEVA cases only revealed previously overlooked or misinterpreted splice-altering SLC26A4 variants in two cases, who are now genetically explained. No deep-intronic or structural variants were identified in any of the M1 subjects. With this study, we have provided important insights that will pave the way for elucidating the missing heritability in M0 and M1 SLC26A4 cases. For pinpointing the pathogenic effect of the CEVA haplotype, additional analyses are required addressing defect(s) at the RNA, protein, or epigenetic level.
© 2021. The Author(s).
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures


References
-
- Andersson R, Gebhard C, Miguel-Escalada I, Hoof I, Bornholdt J, Boyd M, Chen Y, Zhao X, Schmidl C, Suzuki T, Ntini E, Arner E, Valen E, Li K, Schwarzfischer L, Glatz D, Raithel J, Lilje B, Rapin N, Bagger FO, Jørgensen M, Andersen PR, Bertin N, Rackham O, Burroughs AM, Baillie JK, Ishizu Y, Shimizu Y, Furuhata E, Maeda S, Negishi Y, Mungall CJ, Meehan TF, Lassmann T, Itoh M, Kawaji H, Kondo N, Kawai J, Lennartsson A, Daub CO, Heutink P, Hume DA, Jensen TH, Suzuki H, Hayashizaki Y, Müller F, Forrest ARR, Carninci P, Rehli M, Sandelin A, The FC. An atlas of active enhancers across human cell types and tissues. Nature. 2014;507:455–461. doi: 10.1038/nature12787. - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

