Progressive brachial plexus enlargement in hereditary transthyretin amyloidosis
- PMID: 34410494
- PMCID: PMC8940842
- DOI: 10.1007/s00415-021-10754-9
Progressive brachial plexus enlargement in hereditary transthyretin amyloidosis
Abstract
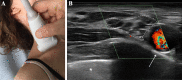
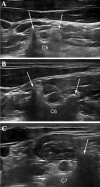
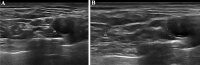
Axonal polyneuropathy is the main feature of hereditary transthyretin amyloidosis (ATTRv). Nerve morphological abnormalities have been reported, but longitudinal changes have never been assessed. We performed a prospective widespread nerve ultrasound evaluation and nerve cross-sectional area (CSA) was compared with baseline data in both ATTRv patients and pre-symptomatic carriers. Thirty-eight subjects were evaluated (mean follow-up 17.1 months), among them 21 had polyneuropathy while 17 were pre-symptomatic carriers. CSA significantly increased at brachial plexus in both groups (p = 0.008 and p = 0.012) pointing to progressive brachial plexus enlargement as a longitudinal biomarker of both disease progression and disease occurrence in pre-symptomatic carriers.
Keywords: Amyloidosis; Brachial plexus; Peripheral nerves; Transthyretin; Ultrasound.
© 2021. The Author(s).
Conflict of interest statement
Alessandro Salvalaggio reports travel grants from Akcea, Alnylam and Pfizer and consulting honoraria from Alnylam. Laura Obici reports speaker and consulting honoraria from Akcea, Alnylam and Pfizer. Marco Luigetti reports financial grants (honoraria and speaking) from Akcea, Alnylam and Pfizer, and travel grants from Pfizer, Kedrion and Grifols. Gulia Bisogni reports financial grants (honoraria and speaking) from Alnylam and travel grants from Pfizer and Grifols. Gian Maria Fabrizi reports consulting honoraria from Akcea and Alnylam, and travel grants from Kedrion and Alnylam. Chiara Gemelli reports travel grants from Akcea and Pfizer. Carlo Martinoli reports financial relationships (consultant, speaker fees, adv board) with Pfizer, Novartis, Sobi, Takeda and Novonordisk. Chiara Briani reports speaker and consulting honoraria from Akcea, Alnylam and Pfizer, and travel grants from Kedrion, Alnylam and CSL Behring. Davide Pareyson reports financial grants (honoraria and speaking) from Alnylam, Akcea, Pfizer, and Inflectis, and travel grants from Kedrion and Pfizer. Silvia Fenu received financial grants (honoraria and speaking) from Alnylam, Akcea, and Pfizer and travel grants from Alnylam and Akcea. Luca Gentile is sub-investigator in clinical trials of Alnylam, Ionis, Takeda and reports travel grants from Kedrion and CSL Behring financial grants (consulting and speaking) from Pfizer.
Figures


References
-
- Coelho T, Maia LF, Martins da Silva A, Waddington Cruz M, Planté-Bordeneuve V, Lozeron P, Suhr OB, Campistol JM, Conceição IM, Schmidt HH, Trigo P, Kelly JW, Labaudinière R, Chan J, Packman J, Wilson A, Grogan DR. Tafamidis for transthyretin familial amyloid polyneuropathy: a randomized, controlled trial. Neurology. 2012;79:785–792. doi: 10.1212/WNL.0b013e3182661eb1. - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials

