Sclerosing Polycystic Adenoma: Conclusive Clinical and Molecular Evidence of Its Neoplastic Nature
- PMID: 34410594
- PMCID: PMC9187789
- DOI: 10.1007/s12105-021-01374-w
Sclerosing Polycystic Adenoma: Conclusive Clinical and Molecular Evidence of Its Neoplastic Nature
Abstract
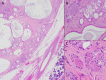
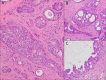
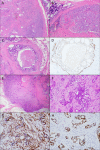
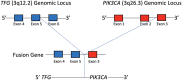
Sclerosing polycystic adenosis, initially considered a non-neoplastic salivary gland lesion and classified as such in the 2017 WHO Classification of Head and Neck Tumors, has been the subject of controversy regarding its possible neoplastic nature. The reporting of recurrent PI3K pathway alteration represents evidence to support these lesions as being neoplastic and more appropriately referred to as sclerosing polycystic adenoma (SPA). Herein, we provide additional evidence that supports the classification of SPA as a true neoplasm. Eight cases of SPA were identified in our database and consultation files. All cases were subjected to PTEN immunohistochemistry (IHC) and next-generation sequencing (NGS). In addition, one patient underwent genetic counseling and germline testing. The cases included 5 men and 3 women with a mean age of 41 years (range 11-78) and all tumors arose in the parotid gland. One patient had multiple recurrences over a period of 2 years. Morphologically the tumors were circumscribed and characterized by an admixture of acini, ducts and cysts embedded in a fibrotic/sclerotic stroma. The cells lining the ducts and cysts showed variable granular, vacuolated, foamy and apocrine cytoplasmic features, as well as acinar cells contained intracytoplasmic brightly eosinophilic granules. The apocrine intraductal proliferations showed mild to moderate atypia in 6 cases. One case showed overt malignant morphology that ranged from intraductal carcinoma to invasive salivary duct carcinoma. Seven cases tested for PTEN IHC showed loss of nuclear expression in the acinar and ductal cells with retained PTEN expression in the myoepithelial cell and stroma. NGS detected PIK3CA or PIK3R1 genetic alterations in 7 cases, including a novel TFG-PIK3CA fusion. Coexisting PTEN mutations were seen in 4 cases, including in a patient with clinical stigmata of Cowden syndrome and confirmed by germline genetic testing. Our findings herein documented including recurrence of tumor, malignant transformation, high prevalence of PI3K pathway oncogenic alterations and the possible heretofore undescribed association with Cowden syndrome add support to classifying SPA as true neoplasms justifying their designation as adenoma, rather than adenosis.
Keywords: Cowden syndrome; PI3K pathway; PTEN; Sclerosing polycystic adenoma; Sclerosing polycystic adenosis.
© 2021. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
All authors declare that they have no conflict of interest as it relates to this research project.
Figures

References
-
- Gnepp DR. Sclerosing polycystic adenosis of the salivary gland: a lesion that may be associated with dysplasia and carcinoma in situ: on: sclerosing polycystic adenosis of parotid gland with dysplasia and ductal carcinoma in situ: report of three cases with immunohistochemical and ultrastructural examination. Skalova A, Michal M, Simpson RHW, et al. Virchows Arch 2002. Adv Anatomic Pathol. 2003;10(4):218–222. doi: 10.1097/00125480-200307000-00005. - DOI - PubMed
-
- Skálová A, Gnepp DR, Simpson RH, Lewis JE, Janssen D, Sima R, et al. Clonal nature of sclerosing polycystic adenosis of salivary glands demonstrated by using the polymorphism of the human androgen receptor (HUMARA) locus as a marker. Am J Surg Pathol. 2006;30(8):939–944. doi: 10.1097/00000478-200608000-00002. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

