Administration of Nivolumab in Metastatic Renal Cell Cancer Following Treatment With mTOR Inhibitors
- PMID: 34410998
- PMCID: PMC8408720
- DOI: 10.21873/invivo.12593
Administration of Nivolumab in Metastatic Renal Cell Cancer Following Treatment With mTOR Inhibitors
Abstract
Background/aim: Immunotherapy with checkpoint inhibitors is currently considered a cornerstone of metastatic renal clear cell cancer (mRCC) therapy. Despite the general improvement in the survival of patients with mRCC, there are some clinical situations that have not been specifically evaluated in clinical trials, such as the use of everolimus before nivolumab.
Patients and methods: We performed a retrospective analysis evaluating the efficacy of nivolumab in the real-world setting, including a subset of patients with previous mTOR inhibitor therapy.
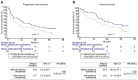
Results: From a total of 56 patients, 25 were pre-treated with everolimus before receiving nivolumab. The overall progression-free survival (PFS), overall survival (OS), and objective response rate were 10.3, 21.3 months, and 34%, respectively. There were no statistically significant differences in patients who were or were not pre-treated with everolimus.
Conclusion: mRCC patients should be treated with checkpoint inhibitors and prior use of mTOR inhibitors should not be a definitive exclusion criterium.
Keywords: Renal cell cancer; mTOR inhibitor; neutrophil-lymphocyte ratio; nivolumab; platelet-lymphocyte ratio; systematic inflammation index.
Copyright © 2021 International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Conflict of interest statement
JK, JKa, AP and HS received honoraria and travel grants from Novartis, Pfizer, Roche, Bristol Myers Squibb, and Ipsen. BM, TB, MZ and IK received honoraria and research support from Novartis, Pfizer, Roche, Bristol Myers Squibb, and Ipsen. OK, JH and MS declare no conflicts of interest in relation to this study.
Figures


References
-
- Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, Castellano D, Choueiri TK, Gurney H, Donskov F, Bono P, Wagstaff J, Gauler TC, Ueda T, Tomita Y, Schutz FA, Kollmannsberger C, Larkin J, Ravaud A, Simon JS, Xu LA, Waxman IM, Sharma P, CheckMate 025 Investigators Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–1813. doi: 10.1056/NEJMoa1510665. - DOI - PMC - PubMed
-
- Heng DY, Xie W, Regan MM, Harshman LC, Bjarnason GA, Vaishampayan UN, Mackenzie M, Wood L, Donskov F, Tan MH, Rha SY, Agarwal N, Kollmannsberger C, Rini BI, Choueiri TK. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol. 2013;14(2):141–148. doi: 10.1016/S1470-2045(12)70559-4. - DOI - PMC - PubMed
-
- Teishima J, Inoue S, Hayashi T, Mita K, Hasegawa Y, Kato M, Kajiwara M, Shigeta M, Maruyama S, Moriyama H, Fujiwara S, Matsubara A. Impact of the systemic immune-inflammation index for the prediction of prognosis and modification of the risk model in patients with metastatic renal cell carcinoma treated with first-line tyrosine kinase inhibitors. Can Urol Assoc J. 2020;14(11):E582–E587. doi: 10.5489/cuaj.6413. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
