Dual-Contrast Biphasic Liver Imaging With Iodine and Gadolinium Using Photon-Counting Detector Computed Tomography: An Exploratory Animal Study
- PMID: 34411033
- PMCID: PMC8732294
- DOI: 10.1097/RLI.0000000000000815
Dual-Contrast Biphasic Liver Imaging With Iodine and Gadolinium Using Photon-Counting Detector Computed Tomography: An Exploratory Animal Study
Abstract
Purpose: The aims of this study were to develop a single-scan dual-contrast protocol for biphasic liver imaging with 2 intravenous contrast agents (iodine and gadolinium) and to evaluate its effectiveness in an exploratory swine study using a photon-counting detector computed tomography (PCD-CT) system.
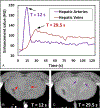
Materials and methods: A dual-contrast CT protocol was developed for PCD-CT to simultaneously acquire 2 phases of liver contrast enhancement, with the late arterial phase enhanced by 1 contrast agent (iodine-based) and the portal venous phase enhanced by the other (gadolinium-based). A gadolinium contrast bolus (gadobutrol: 64 mL, 8 mL/s) and an iodine contrast bolus (iohexol: 40 mL, 5 mL/s) were intravenously injected in the femoral vein of a healthy domestic swine, with the second injection initiated after 17 seconds from the beginning of the first injection; PCD-CT image acquisition was performed 12 seconds after the beginning of the iodine contrast injection. A convolutional neural network (CNN)-based denoising technique was applied to PCD-CT images to overcome the inherent noise magnification issue in iodine/gadolinium decomposition task. Iodine and gadolinium material maps were generated using a 3-material decomposition method in image space. A set of contrast samples (mixed iodine and gadolinium) was attached to the swine belly; quantitative accuracy of material decomposition in these inserts between measured and true concentrations was calculated using root mean square error. An abdominal radiologist qualitatively evaluated the delineation of arterial and venous vasculatures in the swine liver using iodine and gadolinium maps obtained using the dual-contrast PCD-CT protocol.
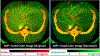
Results: The iodine and gadolinium samples attached to the swine were quantified with root mean square error values of 0.75 mg/mL for iodine and 0.45 mg/mL for gadolinium from the contrast material maps derived from the denoised PCD-CT images. Hepatic arteries containing iodine and veins containing gadolinium in the swine liver could be clearly visualized. Compared with the original images, better distinctions between 2 liver phases were achieved using CNN denoising, with approximately 60% to 80% noise reduction in contrast material maps acquired with the denoised PCD-CT images compared with the original images.
Conclusions: Simultaneous biphasic liver imaging in a single multienergy PCD-CT acquisition using a dual-contrast (iodine and gadolinium) injection protocol and CNN denoising was demonstrated in a swine study, where the enhanced hepatic arteries (containing iodine) and the enhanced hepatic veins (containing gadolinium) could be clearly visualized and delineated in the swine liver.
Copyright © 2021 Wolters Kluwer Health, Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest and sources of funding: Research reported in this publication was supported by the National Institutes of Health under award numbers R21 EB024071, R01 EB016966, R01 EB028590, and C06 RR018898. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The device described is a research scanner and not commercially available. Dr McCollough and Dr Fletcher receive industry grant support from Siemens. No other potential conflicts of interest were declared.
Figures



References
-
- Oliver JH 3rd, Baron RL. Helical biphasic contrast-enhanced CT of the liver: technique, indications, interpretation, and pitfalls. Radiology. 1996;201:1–14. - PubMed
-
- Xie B, Su T, Kaftandjian V, et al. Material decomposition in x-ray spectral CT using multiple constraints in image domain. J Nondestruct Eval. 2019;38:16.
-
- Lu Y, Kowarschik M, Huang X, et al. A learning-based material decomposition pipeline for multi-energy x-ray imaging. Med Phys. 2019;46:689–703. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

